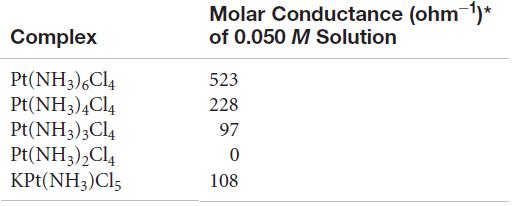
Based on the molar conductance values listed here for the series of platinum(IV) complexes, write the formula
Question:
Based on the molar conductance values listed here for the series of platinum(IV) complexes, write the formula for each complex so as to show which ligands are in the coordination sphere of the metal. By way of example, the molar conductances of 0.050 M NaCl and BaCl2 are 107 ohm-1 and 197 ohm-1, respectively.
Transcribed Image Text:
Molar Conductance (ohm 1)* Complex of 0.050 M Solution Pt(NH3),Cl4 Pt(NH3),Cl4 Pt(NH3)3Cl4 Pt(NH3),Cl4 KPt(NH3)Cl5 523 228 97 108
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The molar conductance values of the series of platinumIV complexes suggest that the number of ions p...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere: (a) Hexaamminechromium (III) nitrate (b) Tetraamminecarbonatocobalt (III)...
-
The Eo values for two low-spin iron complexes in acidic solution are as follows: (a) Is it thermodynamically favorable to reduce both Fe(III) complexes to their Fe(II) analogs? Explain. (b) Which...
-
The conductance G of an object is defined as the reciprocal of the resistance R; that is, G = 1/R. The unit of conductance is a mho (= ohm-1), which is also called the siemens (S). What is the...
-
1. Consider the linear system Ax = b (all integer values) with the notation shown below, 1 -3 -1 -2 - H 5 7 - 2261x11 X2 -4 3 9 16x3 615 LX4- = A X b The element a24 = a has been lost. Assume,...
-
Derive an expression analogous to Equation 26-18 for the titration of M+ (concentration C0M, volume = V0M ) with X- (titrant concentration = C0X ). Your equation allows you to compute the volume of...
-
If an appointment system evenly spaces out appointments throughout the day, then patients arriving during the second half of the day can expect to wait the same amount of time as patients arriving...
-
Let $y_{i j}$ for $i=1, \ldots, b$ and $j=1, \ldots, k$ be the measurement for the unit assigned to the $j^{\text {th }}$ treatment in the $i^{t h}$ block. a. Show that \[S S_{T}=S S_{\text {Treat...
-
The balance sheet items for The Oven Bakery (arranged in alphabetical order) were as follows at August 1, 2011. (You are to compute the missing figure for Retained Earnings.) During the next two...
-
A rainfall record contains 44 years of rainfall measurement at 10 minute intervals. The maximum rainfall amounts for intervals of 10 min and 20 min, 30 min, 40 min, 50 min, and 60 min have been...
-
According to the expectations theory, which of the following is closest to the one-year implied forward rate one year from now? a. 6.58 percent b. 5.75 percent c. 6.25 percent James Wallace, CFA, is...
-
(a) Sketch a diagram that shows the definition of the crystal-field splitting energy () for an octahedral crystal field. (b)What is the relationship between the magnitude of and the energy of the...
-
Sketch the structure of the complex in each of the following compounds and give the full compound name: (a) cis-[Co(NH 3 )4(H 2 O) 2 ](NO 3 ) 2 (b) Na 2 [Ru(H 2 O)Cl 5 ] (c) trans-NH 4 [Co(C 2 O 4 )...
-
In problem, construct a polynomial function f with the given characteristics. Zeros: -5 (multiplicity 2); 2 (multiplicity 1); 4 (multiplicity 1); degree 4; contains the point (3, 128)
-
True Or False Negligence is the most common tort, whereas intentional torts are rarely encountered in practice.
-
True Or False It is harder to prove that a defendant is guilty of a crime than to prove that they are liable for a tort.
-
A tort that involves the pleading of vi et armis is a(n) _______________ _______________ _______________. A more restrictive tort that allows recovery in the absence of a showing of force is a(n)...
-
True Or False In a jury trial, all issues are decided by the jury.
-
__________________ torts require no intent or negligence.
-
Mio was transfer from New York to Germany. He lived and worked in Germany for 340 days in 2015. Mio's salary for 2015 is $190,000. In your computation, round any division to four decimal places...
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
For the electric dipole shown in Fig. 4-13, d =1 cm and |E| = 4 (mV/m) at R = 1 m and 0o. Find E at R = 2 m and 90 o.
-
For each of the following distributions of the electric potential V, sketch the corresponding distribution of E (in all cases, the vertical axis is in volts and the horizontal axis is in meters):
-
Given the electric field find the electric potential of point A with respect to point B where A is at + 2 m and B at 4 m, both on the z-axis. E = R (V/m). R2 18
-
We know that the root loci start at the poles (for K = 0) and end at the zeros (for K = o) of P(s). Also, we know that the root loci are on the real axis to the left of the odd numbered poles and...
-
4. For the cam displacement below with maximum rise of 25 mm, design a disk cam with knife edge follower graphically. Assume that the cam rotates in clockwise direction. Let the minimum diameter of...
-
Consider a Carnot heat engine placed between a finite thermal energy source and an infinite thermal energy sink. Since the temperature of the thermal source is constantly changing, then so must the...

Study smarter with the SolutionInn App


