Given the following bond-dissociation energies, calculate the average bond enthalpy for the Ti-Cl bond. AH(kJ/mol) TiCl4(g) TiCl;(g)
Question:
Given the following bond-dissociation energies, calculate the average bond enthalpy for the Ti-Cl bond.
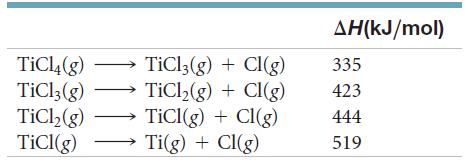
Transcribed Image Text:
AH(kJ/mol) TiCl4(g) TiCl;(g) TİC,(g) TICI(g) TiCl3(g) + Cl(g) TİCI,(g) + CI(g) TiCI(g) + CI(g) Ti(g) + CI(g) 335 423 444 519
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Average bond energy of TiC1 bond TiC1 4 g TiC1 3 ...View the full answer

Answered By

Umair Expert
Hi Everyone.
I have 6 years of teaching experience.
I am serving as a tutor 2 more websites.
I will provide you projects and solutions of questions related to any subject.
I am a good programmer.(PHP, Python)
I am good command in mathematics.
I am here to assist you.
I'll be very happy to work with you...
Thanks dear..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF (CH3)2CHF + H2O (b)...
-
a. Propose a mechanism for the following reaction: b. Given that the Ho value for the reaction is -42 kcal/mol and the bond dissociation energies for the C--H, C--Cl and O--H bonds are 101, 82, and...
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
What other types of contingency planning should Matt and Chris include to make the report comprehensive? Please explain the relevance of each suggestion.
-
If the double-stem display still has too few stems, we may wish to construct a stem and-leaf display with a separate stem to hold leaves 0 and 1, 2 and 3, 4 and 5, 6 and 7, and a stem to hold 8 and...
-
Why is profit not a suitable measure of divisional performance.
-
As a kid, Haley Rosen played all the sports but quickly fell in love with soccer. She played soccer through her school years and was skilled at the game. When it was time for college, Stanford...
-
Edwards Manufacturing Company (EMC) is considering replacing one machine with another. The old machine was purchased 3 years ago for an installed cost of $10,000. The firm is depreciating the machine...
-
1. Find the focal length of a convex mirror of radius of curvature 1m. 2. Focal length of a convex mirror is 50 cm. What is its radius of curvature? 3. Radius of curvature of a concave mirror is 25...
-
The Income Statement columns of the work sheet of Redfax Company for the fiscal year ended December 31 follow. During the year, D. Redfax withdrew $12,000. Journalize the closing entries. INCOME...
-
Which of the following bonds are polar: (a) B - F (b) Cl Cl (c) Se O (d) H I Which is the more electronegative atom in each polar bond?
-
(a) If these three balloons are all the same size, what angle is formed between the red one and the green one? (b) If additional air is added to the blue balloon so that it gets larger, what happens...
-
Think of a product you purchased recently after seeing an advertisement. Which brand strategies can you discern in the advertising?
-
What is the standard in the law for the court to determine who will have parenting time or custody of a child and decision making authority?
-
The regular price of a photograph printed on a canvas is $18. You have a coupon for 15% off. How much is the discount?
-
Do families have to be legally married in order to experience "family violence" under the law? NO Why?
-
Identify the population and the sample in the experiment described below. A survey of nearly 500 college students from across the United States shows that the average credit card debt of college...
-
Using the Indiana Code section that discusses our state's custody provisions. Define joint custody? Is joint custody a permissible option? If yes, is there a presumption in favor of joint custody?...
-
An electron and a proton have the same speed. Ignore relativistic effects and determine the ratio electron/ proton of their de Broglie wavelengths.
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
Consider subsonic flow in a converging nozzle with fixed inlet conditions. What is the effect of dropping the back pressure to the critical pressure on? (a) The exit velocity, (b) The exit pressure,...
-
Consider gas flow through a converging nozzle with specified inlet conditions. We know that the highest velocity the fluid can have at the nozzle exit is the sonic velocity, at which point the mass...
-
How does the parameter Ma* differ from the Mach number Ma?
-
Using quantitative risk analysis how do we determine ALE (Annual Loss Expectancy)? Choose one 1 point Single Loss Expectancy x Exposure Factor = Annual Loss Expectancy (ALE) Annual Loss Expectancy...
-
If Lena deposited $ 1 comma 000 in cash into a chequing account, Part 2 A. Upper M 1 plus increased and Upper M 2 plus decreased. B. M1+ decreased and M2+ increased. C. Upper M 1 plus and Upper M 2...
-
At December 31 Assets Cash Accounts receivable, net Merchandise inventory Prepaid expenses Plant assets, net Total assets Liabilities and Equity Accounts payable Long-term notes payable Common stock,...

Study smarter with the SolutionInn App


