In 2010, a team of scientists from Russia and the U.S. reported creation of the first atom
Question:
In 2010, a team of scientists from Russia and the U.S. reported creation of the first atom of element 117, which is not yet named and is denoted [117]. The synthesis involved the collision of a target of 24997Bk with accelerated ions of an isotope which we will denote Q. The product atom, which we will call Z, immediately releases neutrons and forms 294117[117] :
24997Bk + Q → Z → 294117[117] + 310n
(a) What are the identities of isotopes Q and Z?
(b) Isotope Q is unusual in that it is very long-lived (its half-life is on the order of 1019 yr) in spite of having an unfavorable neutron-to-proton ratio (Figure 21.2). Can you propose a reason for its unusual stability?
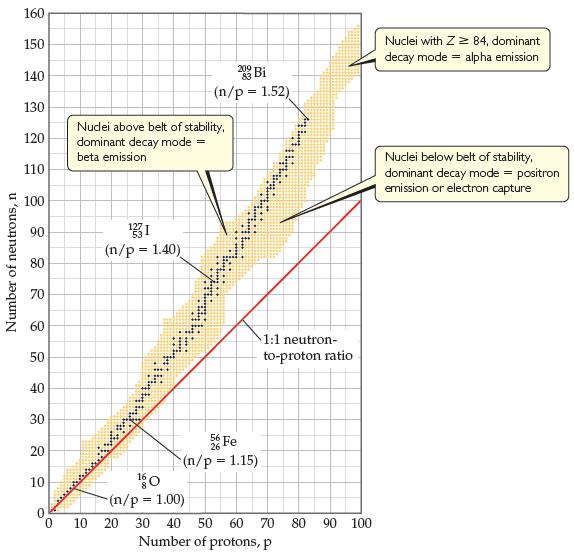
(c) Collision of ions of isotope Q with a target was also used to produce the first atoms of livermorium, Lv. The initial product of this collision was 296116Lv. What was the target isotope with which Q collided in this experiment?
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





