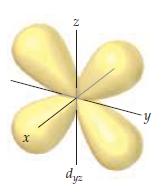
The accompanying drawing shows a contour plot for a d yz orbital. Consider the quantum numbers that
Question:
The accompanying drawing shows a contour plot for a dyz orbital. Consider the quantum numbers that could potentially correspond to this orbital.
(a) What is the smallest possible value of the principal quantum number, n?
(b) What is the value of the angular momentum quantum number, l?
(c) What is the largest possible value of the magnetic quantum number, ml?
(d) The probability density goes to zero along which of the following planes: xy, xz, or yz?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





