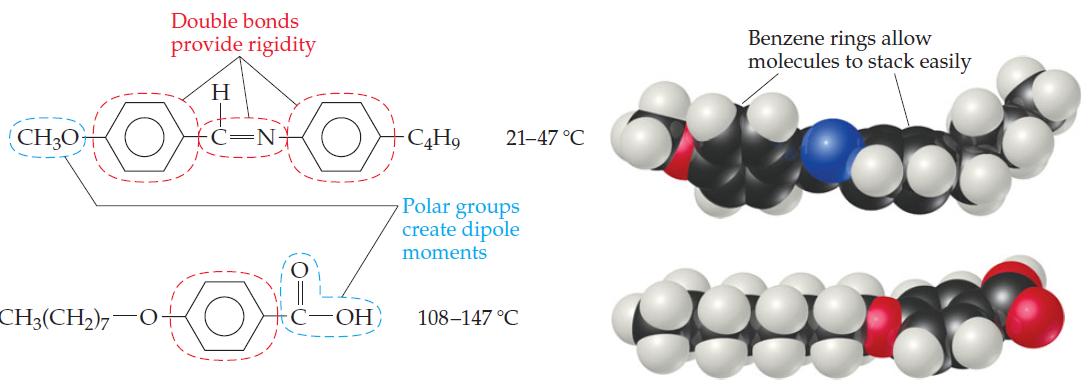
The molecules shown in Figure 11.33 possess polar groups (that is, groupings of atoms that give rise
Question:
The molecules shown in Figure 11.33 possess polar groups (that is, groupings of atoms that give rise to sizable dipole moments within the molecules).How might the presence of polar groups enhance the tendency toward liquid crystal formation?
Figure 11.33
Transcribed Image Text:
Double bonds provide rigidity Benzene rings allow molecules to stack easily H (CH3O+ 21-47 °C Polar groups create dipole moments CH3(CH2),-O+ C-OH 108-147 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
The presence of polar groups in molecules can enhance the tendency toward liq...View the full answer

Answered By

Ravi Tomar
I have 5 years of experience as an Agricultural Economics tutor. During this time, I have been able to successfully provide guidance to students in their studies and help them develop their knowledge and understanding of the subject. My approach to teaching has always been to combine academic learning with practical application, often drawing on my professional experience to help students better understand how the concepts they learn apply to the real world. I also focus on helping students develop critical thinking skills, enabling them to tackle problems independently and develop their own solutions. I have also been able to provide support on specific assignments, helping students to structure their work and ensure that it meets the required quality and standards.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
A hypothetical phase diagram is shown in Figure 11-26. (a) Are there any inter metallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric.
-
The Cu-Zn phase diagram is shown in Figure 11-27. (a) Are any inter metallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric.
-
If we assume that the energy-level diagrams for homo nuclear diatomic molecules shown in Figure 9.43 can be applied to hetero nuclear diatomic molecules and ions, predict the bond order and magnetic...
-
1. What is Ladures target market and retail strategy in the United States? 2. Explain the reasons Ladure owns its stores in some countries and uses franchising with local licensees in others. 3....
-
A person with asthma took measurements by blowing into a peak-flow meter on seven consecutive days. Display the data in a dot diagram. 429 425 471 422 432 444 454
-
Do piano lessons improve the spatial-temporal reasoning of preschool children? A study designed to investigate this question measured the spatial-temporal reasoning of a random sample of 34 preschool...
-
Generalized Regression Techniques and Variable Selection In Chapter 9, we introduced generalized regression techniques as an approach to handling the multicollinearity problem. The LASSO can...
-
Judy is the production manager of Test Images, a division of the larger corporation, Image View, Inc. Judy has complained several times to the corporate office that their cost reports used to...
-
Instructions Assuming that the directors decide to declare total dividends in the amount of $366,000, determine how much each class of stock should receive under each of the conditions stated below....
-
X in State A and Y in State B plan to enter into a contract. What can they do to avoid the impact of fluctuations in the value of their money of account?
-
Briefly explain the significance of the constants a and b in the van der Waals equation.
-
Ethylene glycol (HOCH 2 CH 2 OH) is the major component of antifreeze. It is a slightly viscous liquid, not very volatile at room temperature, with a boiling point of 198C. Pentane (C 5 H 12 ), which...
-
Cole, a 48-year-old married individual, received the following in 2017: S corporation stock dividends ............................................................ $5,000 Interest earned on federal...
-
Do you agree with the hypothesis presented by the Mean World Index? Why or why not?
-
Do you agree with Fisher that humans are storytellers? What does that mean to you in a practical sense?
-
What do you think about phenomenology as a method for discovering what marginalized people really think about their communication strategies? What do you think might be the pitfalls in asking people...
-
If all truths are understood as coming from some subjective standpoint, how is it possible for people to communicate? If there is no objective truth, how do we reach agreement among people with...
-
Can you coin terms for experiences that women have and men do not? What about the reverse? Do you agree with MGT that the English language fits the male experience of the world better than the female...
-
Sophia and Jacob are married and file a joint return. The return for 2014 included a Form 2106 for each of them. The return for 2015, however, included a Form 2106 and a Schedule C. In terms of...
-
Following is the current balance sheet for a local partnership of doctors: The following questions represent independent situations: a. E is going to invest enough money in this partnership to...
-
Reconsider Prob. 1791. Determine the downstream Mach number, pressure, and temperature below the wedge for a strong oblique shock for an upstream Mach number of 5.
-
Air at 8 psia, 20F, and a Mach number of 2.0 is forced to turn upward by a ramp that makes an 8 angle off the flow direction. As a result, a weak oblique shock forms. Determine the wave angle, Mach...
-
Air flowing at P1 = 40 kPa, T1 = 280 K, and Ma1 = 3.6 is forced to undergo an expansion turn of 15. Determine the Mach number, pressure, and temperature of air after the expansion.
-
Annuity Payments Go to www.fcfcorp.com/onlinecalc.htm. Use the calculator to solve this problem. If you have $1,500,000 when you retire and want to withdraw an equal amount for the next 30 years, how...
-
Calculating Future Values Go to www.dinkytown.net and follow the Savings Calculator link. If you currently have $10,000 and invest this money at 9 percent, how much will you have in 30 years? Assume...
-
In 2023, Amanda and Jaxon Stuart have a daughter who is 1 year old. The Stuarts are full-time students and are both 23 years old. Their only sources of income are gains from stock they held for three...

Study smarter with the SolutionInn App


