The table below shows the normal boiling points of benzene and benzene derivatives. (a) How many of
Question:
The table below shows the normal boiling points of benzene and benzene derivatives.
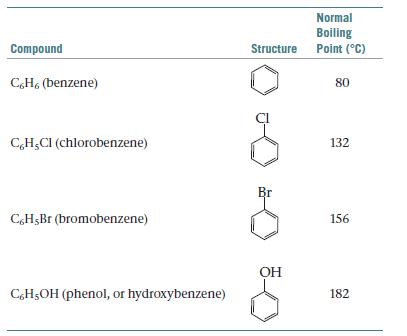
(a) How many of these compounds exhibit dispersion interactions?
(b) How many of these compounds exhibit dipole-dipole interactions?
(c) How many of these compounds exhibit hydrogen bonding?
(d) Why is the boiling point of bromobenzene higher than that of chlorobenzene?
(e) Why is the boiling point of phenol the highest of all?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





