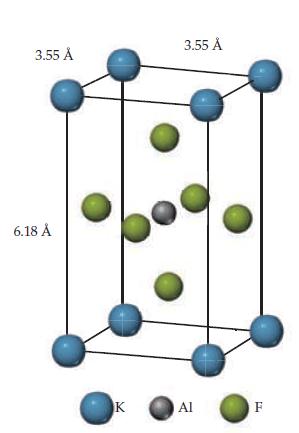
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type
Question:
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here.
(a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)?
(b) What is the empirical formula?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





