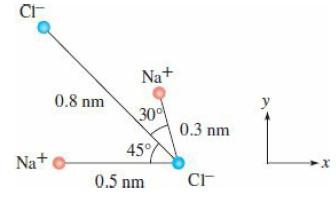
What is the electric force on the chloride ion in the lower righthand corner in the diagram?
Question:
What is the electric force on the chloride ion in the lower righthand corner in the diagram? Since the ions are in water, the “effective charge” on the chloride ions (Cl−) is −2 × 10−21 C and that of the sodium ions (Na+) is +2 × 10−21 C. (The effective charge is a way to account for the partial shielding due to nearby water molecules.) Assume that all four ions are coplanar.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:





