A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash
Question:
A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash tank.
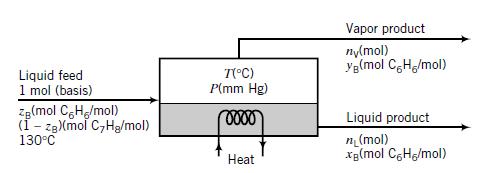
The pressure in the unit may be adjusted to any desired value, and the heat input may similarly be adjusted to vary the temperature at which the separation is conducted. The vapor and liquid product streams both emerge at the temperature (°C) and pressure (mm Hg) maintained in the vessel.
Assume that the vapor pressures of benzene and toluene are given by the Antoine equation, Table 6.1-1; that Raoult’s law—Equation 6.4-1—applies; and that the enthalpies of benzene and toluene liquid and vapor are linear functions of temperature. Specific enthalpies at two temperatures are given here for each substance in each phase.

(a) Suppose the feed is equimolar in benzene and toluene (zB = 0 500). Take a basis of 1 mol of feed and do the degree-of-freedom analysis on the unit to show that if and are specified, you can calculate the molar compositions of each phase (xB and yB), the moles of the liquid and vapor products (nL and nV), and the required heat input (Q). Don’t do any numerical calculations in this part.
(b) Do the calculations of part (a) for T = 90°C and P = 652 mm Hg. (Suggestion: First derive an equation for xB that can be solved by trial and error from known values of T and P.)
(c) For zB = 0.5 and T = 90°C, there is a range of feasible operating pressures for the evaporator, Pmin < P < Pmax. If the If the evaporator pressure P fell outside this range, no separation of benzene and toluene would be achieved. Why not? What would emerge from the unit if P < Pmin? What would emerge if P > Pmax?
(d) Set up a spreadsheet to perform the calculation of part (b) and then use it to determine Pmax and Pmin. The spreadsheet should appear as follows (some solutions are shown):
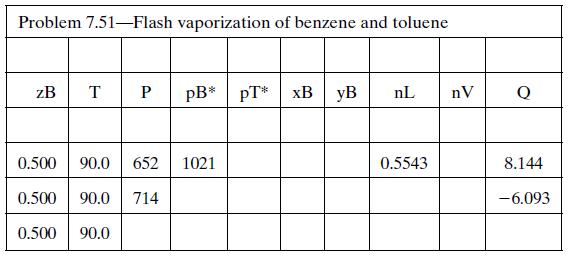
Additional columns may be used to store other calculated variables (e.g., specific enthalpies). Briefly explain why Q is positive when P = 652 mm Hg and negative when P = 714 mm Hg.
(e) In successive rows, repeat the calculation for the same zB and T at several pressures between
Pmin and Pmax. Generate a plot (using the spreadsheet program itself, if possible) of nv versus P. At approximately what pressure is half of the feed stream vaporized?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





