In the waste liquor neutralizer, both the hydrochloric acid separated from the wetcake in the reactor centrifuge
Question:
In the waste liquor neutralizer, both the hydrochloric acid separated from the wetcake in the reactor centrifuge and the CO2 generated in the resin neutralizer are neutralized with an aqueous solution containing 10 wt% NaOH and also some dissolved NaCl and Na2CO3.
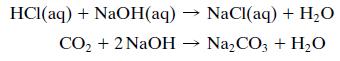
The exit stream from the neutralizer joins with the effluent liquor from the product centrifuge. Most of the combined stream is fed to a waste treatment facility, but a portion of it is fed to the NaOH makeup tank (a batch stirred tank) to prepare more caustic feed solution for the neutralizer. Enough NaOH(s) is added to the makeup tank to bring the solution concentration back up to 10 wt% NaOH.
Each batch prepared in this manner contains enough solution to supply the waste liquor neutralizer for eight hours of operation (one shift). When the NaOH pellets have completely dissolved and a batch is ready, a valve in the tank exit line is opened and the batch is fed by gravity to the NaOH feed drum, from which solution is pumped continuously to the waste liquor neutralizer.
(a) Draw and label a flowchart for this part of the process.
(b) Speculate on the reason that the HCl must be neutralized.
(c) Calculate the required mass flow rate (kg/h) and composition (component mass fractions) of the caustic solution fed to the neutralizer, the mass flow rate and composition of the solution leaving the neutralizer, and the fraction of the combined salt solution sent to the makeup tank.
(d) Calculate (i) the mass of solid NaOH (kg) required per batch of solution prepared in the tank, and (ii) the required tank size if the specific gravity of the exiting solution is 1.11 and a batch occupies 60% of the total tank volume.
(e) Determine the metric tons of NaCl and Na2CO3 that must be processed each year in the waste treatment facility. (Recall that the plant operates 300 days per year.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





