Vapor pressure data for chlorine are given below. a. Use these data and the ClausiusClapeyron equation (Equation
Question:
Vapor pressure data for chlorine are given below.
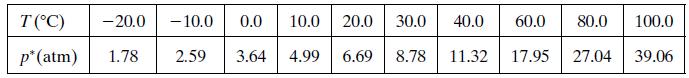
a. Use these data and the Clausius–Clapeyron equation (Equation 6.1-3) to estimate the heat of vaporization of chlorine (kJ/mol) and to obtain an expression for P*Cl2 (T).
b. What is the operating pressure in the chlorine vaporizer (torr)?
c. At what rate (kW) must heat be added to the chlorine in the vaporizer?
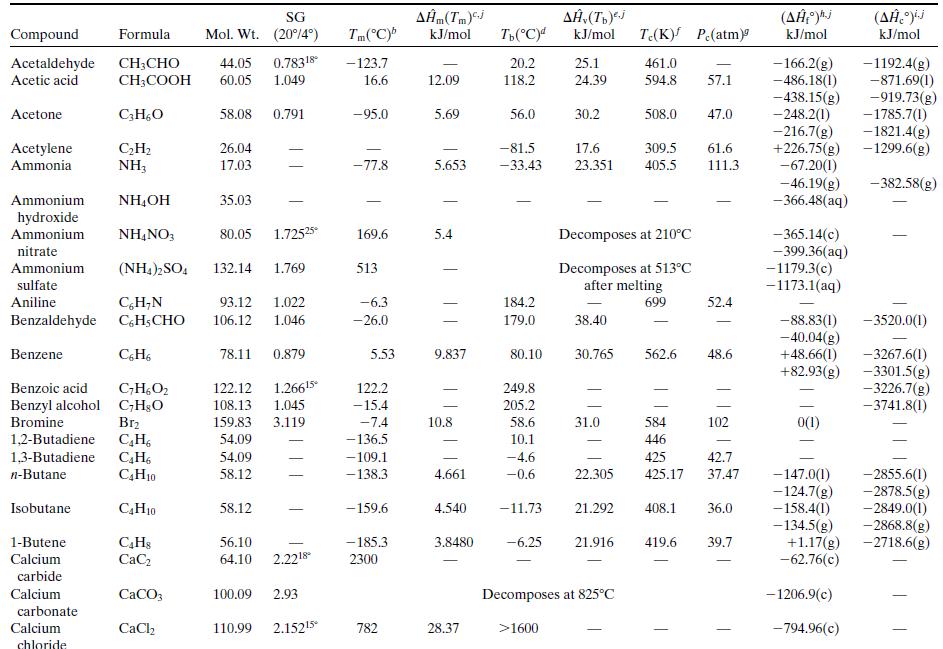
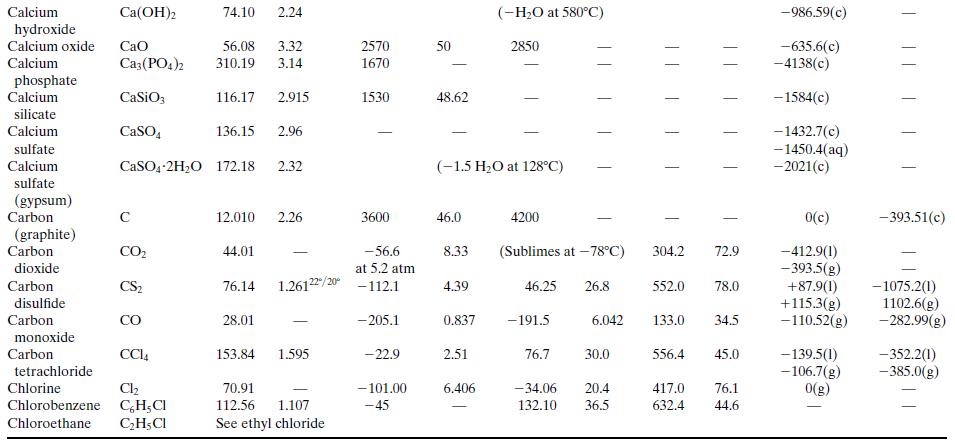
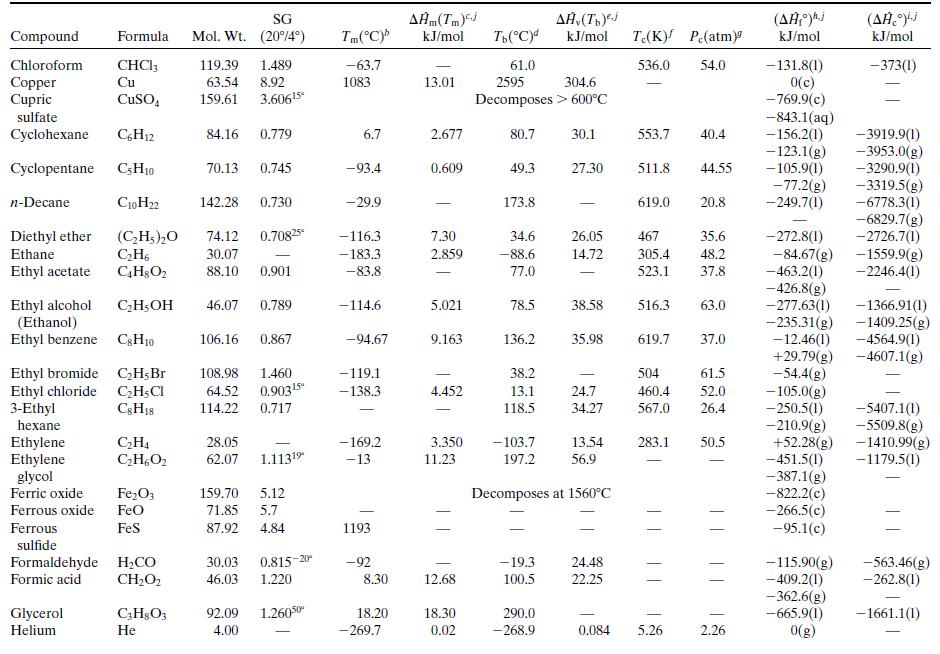
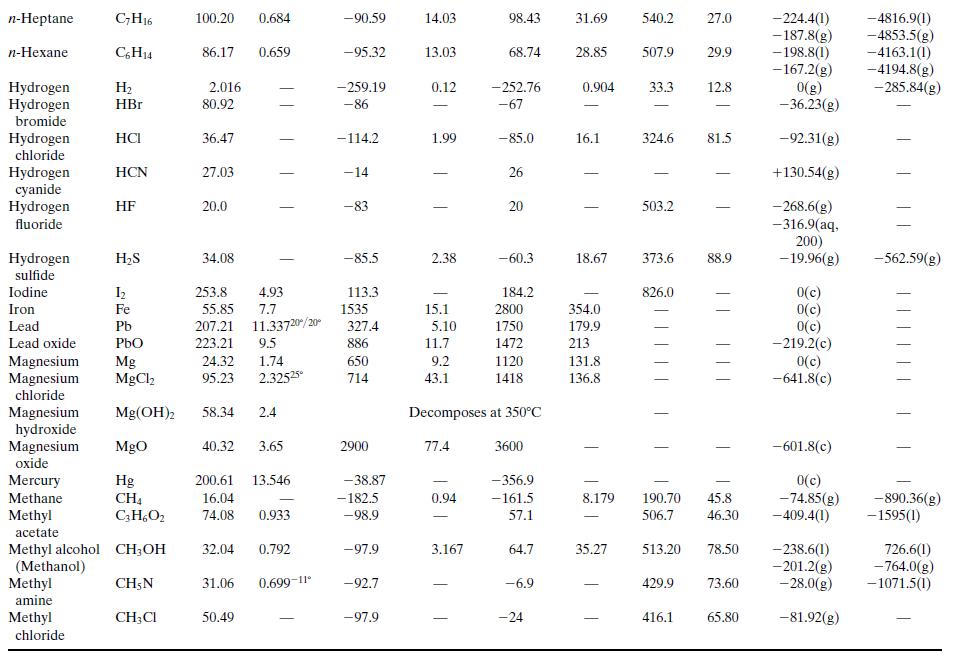
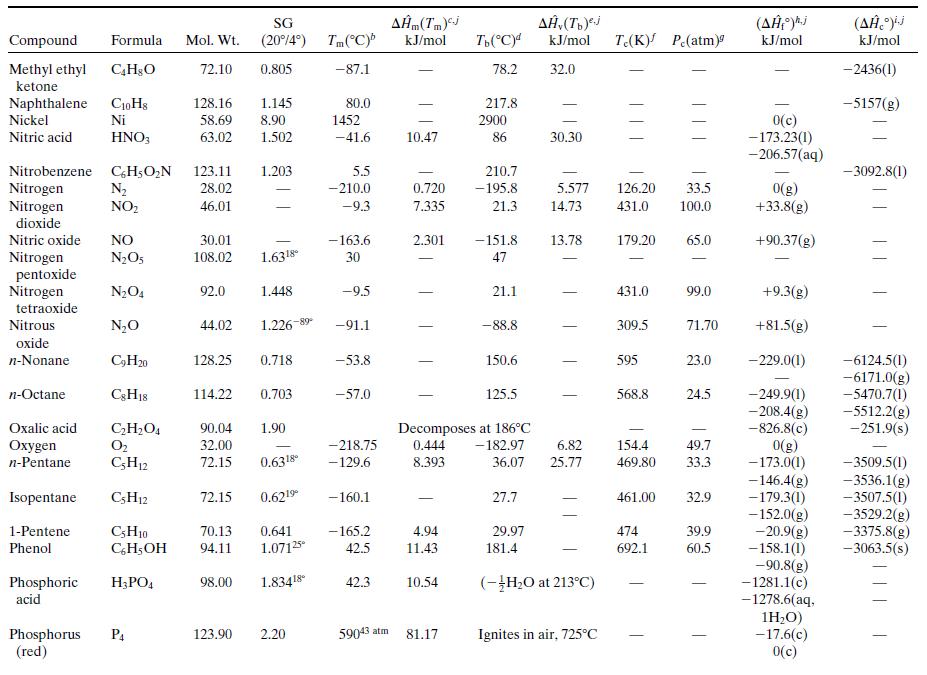
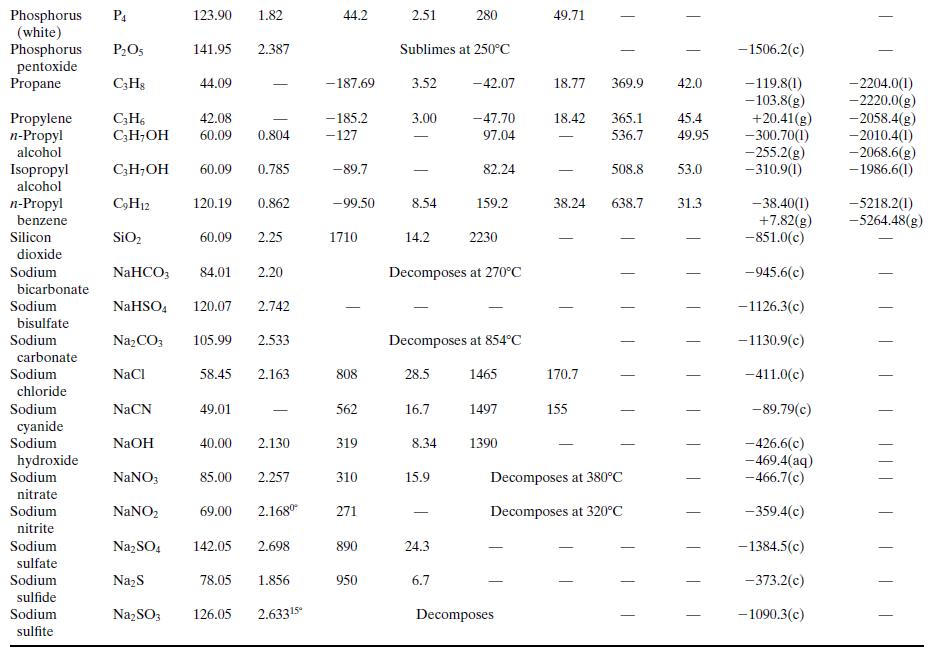
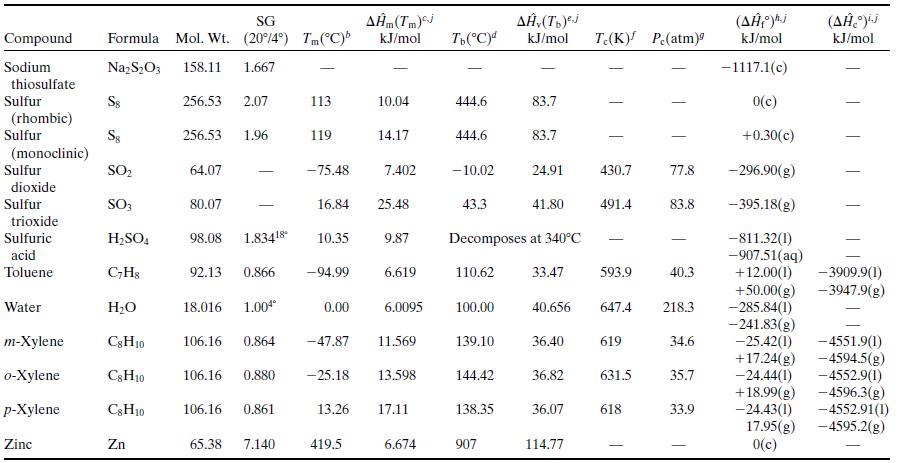
What is the percentage difference between the calculated value of ΔĤv and the one given in Table B.1?Why might the two values differ? (Think of several possible reasons.)
Table B.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





