Question:
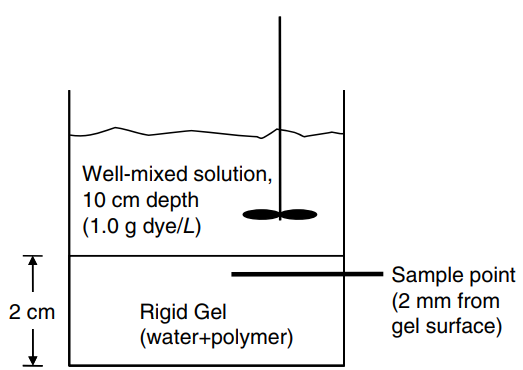
A simple experiment is set up to measure the effective diffusion coefficient of blue dextran dye into a rigid agarose gel. An aqueous, well-mixed solution of liquid height 10 cm con taining 1.0 g/L (1.0 × 10
-3g/cm
3) of the dye rests over the rigid gel of 2.0 cm thickness, as shown in the figure in the right-hand column above. The solubility of the dye in both water and the agarose gel are the same€”i.e., the concentration of dye on the water side of the water-gel interface is equal to the concentration of dye on the gel side of the water-gel interface. The aqueous gel is generally considered a water-hydrated poly mer, where solute molecules diffuse through the hydrated regions. Initially, there is no dye dissolved the gel. After 24 h, a tiny section of the gel, 2.0 mm from the surface, is very carefully excised with a syringe needle. The concentration of the dye within the gel sample is measured by a spectrometer, and reads 0.203 g dye/L (2.03 × 10
-4g/cm
3). Based on the experimental measurements, estimate the effective diffusion coefficient of blue dextran dye in the agarose gel. Justify by a calculation that it is reasonable to assume that the concentration of the dye in the well-mixed aqueous solution does not change significantly over the course of the experiment.
Transcribed Image Text:
Well-mixed solution, 10 cm depth (1.0 g dye/L) Sample point (2 mm from gel surface) Rigid Gel (water+polymer) 2 cm








