An open well contains water contaminated with volatile benzene at the bottom of the well, with dimensions
Question:
a. Define the source, sink, and system boundary for all of the species undergoing mass transfer. State three reasonable assumptions that describe the mass-transfer process.
b. State reasonable boundary conditions, and specify their numerical values with units, for all of the species undergoing mass transfer.
c. What are the maximum emission rates (in mole/day) of benzene and water vapor from the well? What is cumulative benzene emission (in grams) over a period of 30 days? Is it significant?
Potentially useful data: The molecular diffusion coefficient of benzene in dissolved water is 1.1 × 10-5 cm2/s at 25°C, and the molecular diffusion coefficient of benzene vapor in air is 0.093 cm2/s at 1.0 atm and 25°C. The Henry€™s law constant for benzene partitioning into water is H = 4.84 × 10-3 m3 · atm/mole at 25°C. The vapor pressure of liquid water is 0.0317 bar (0.031 atm) at 25°C, and the density is 1000 kg/m3 at 25°C. The vapor pressure of benzene is 0.13 atm at 25°C. The humidity of the air flowing over the well hole is 40% of relative saturation at 25°C. The molecular weight of benzene is 78 g/mole.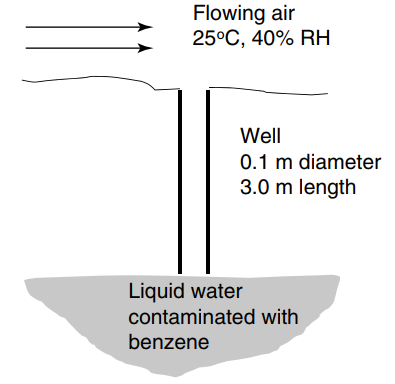
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





