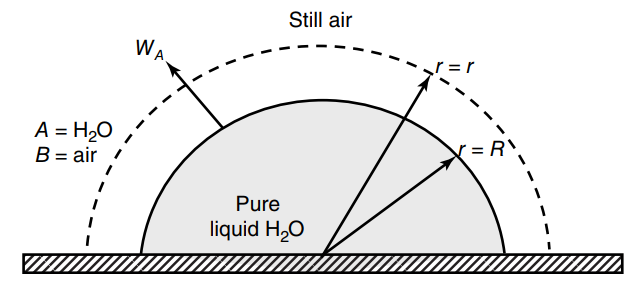
Consider a hemispherical droplet of liquid water residing on a flat surface, as shown in the figure
Question:
a. What is the total evaporation transfer rate (WA) of water from a water droplet of radius 5.0mm in units of mmole H2O per hour?
b. Determine the time it will take for the water droplet to completely evaporate at 30C and 1.0 atm total system pressure if the initial droplet radius is 5.0 mm.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





