Consider the process shown in the figure. A bulk gas stream containing 0.10 mole% of carbon monoxide
Question:
a. What are the Schmidt numbers for CO and O2 mass transfer? What species (CO, O2, CO2) is considered the carrier gas?
b. For CO mass transfer, what is the average convective mass- transfer coefficient (kc) over the 0.50 m length of the catalytic surface, and the local mass transfer coefficient (kc,x) at the far edge of the catalytic surface (x = L = 0.50 m)?
c. Using boundary-layer theory, scale kc for CO mass transfer to kc for O2 transfer.
d. At 600 K, the surface reaction constant for the first-order oxidation reaction with respect to CO concentration is ks = 1.5 cm/s. What is the average molar flux of CO to the catalytic surface, assuming that the composition of CO in the bulk gas is maintained at 0.10 mole%?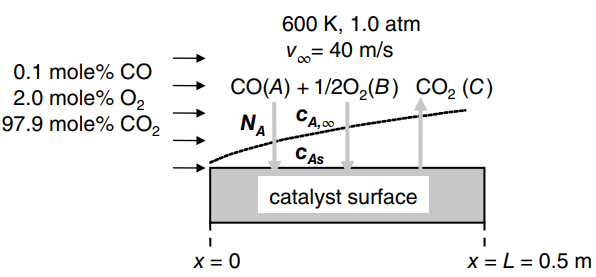
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





