In the distillation of a benzene/toluene mixture, a vapor richer in the more volatile component benzene is
Question:
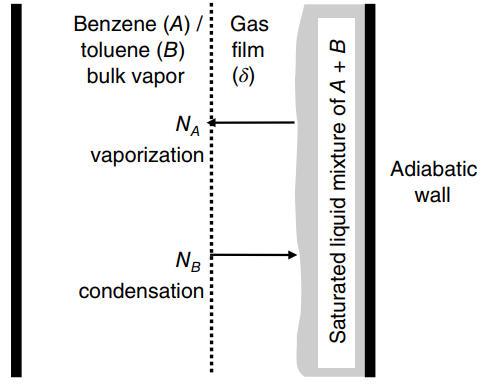
heats of vaporization of benzene (A) and toluene (B) are 30 and 33 kJ/gmole, respectively. Both components are diffusing through a gas film of thickness δ. Develop the final, integrated form of Fick€™s flux equation to predict the steady-state mass transfer of benzene through the gas film. The equation must include terms for the bulk gas-phase mole fraction of benzene, the gas-phase mole fraction of benzene in equilibrium with the liquid solution, the diffusion coefficient of benzene in toluene, the diffusion path δ, and the total molar gas concentration, and the latent heats of vaporization for benzene (ΔHv,A) and toluene (ΔHv,B). Assume that distillation is an adiabatic process.
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





