It is desired to recover hexane vapor (solute A) from air using an absorption process. The absorption
Question:
a. What is the overall mass-transfer coefficient based on the liquid phase, KL, and molar flux NA?
b. What is the composition of hexane at the gas€”liquid inter face, in terms of pA,i and xA,i?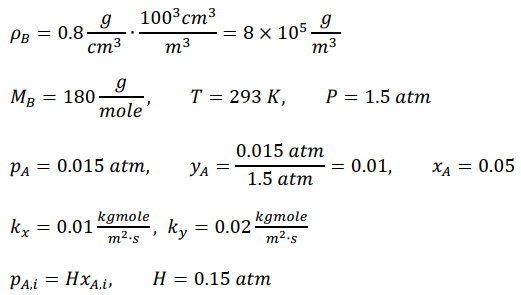
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





