It is desired to remove 83.3% of dissolved NH 3 from an aqueous stream containing 10.7 mole%
Question:
a. Plot out the equilibrium line in mole ratio coordinates, YAvs. XA. What is the maximum possible mole fraction and mole ratio of NH3in the outlet air stream, and the minimum possible air flow rate into the tower?
b. What is the operating air molar flow rate and mole faction of NH3in the outlet gas at 1.5 times the minimum molar flow rate of air? Plot out the operating line in mole ratio coordinates. Is the operating line above or below the equilibrium line?
Equilibrium distribution data for NH3-water at 30°C (A = NH3):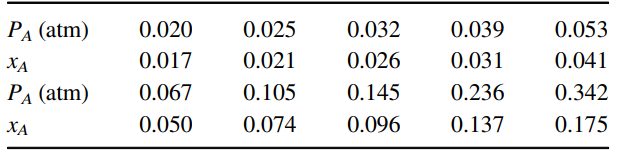
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





