Ozone gas (O 3 , solute A) dissolved in high-purity water is commonly used in wet cleaning
Question:
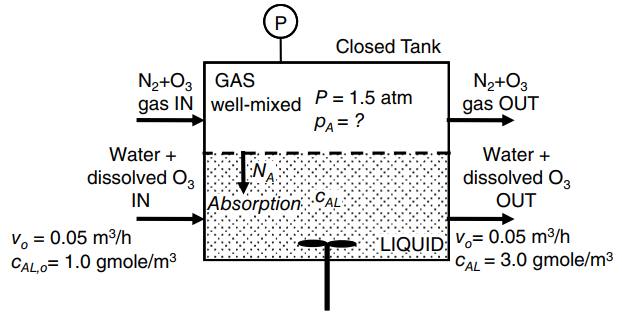
a. What are m, and the Henry€™s law constant H in units of atm? Is O3 very soluble in water?
b. What is the overall mass-transfer coefficient KG, based on the overall gas-phase driving force?
c. What is the overall mass-transfer coefficient KL based on the overall liquid-phase driving force?
d. For the process to operate as intended, what are the required partial pressure (pA) and mole fraction (yA) of ozone (O3) in the gas phase inside the tank? As part of your solution,
develop a material balance model in algebraic form for solute A that contains the following terms: v0, volumetric flow rate of liquid (m3/hr); cAL,0, inlet concentration of solute A in liquid (gmole A/m3); cAL, outlet concentration of solute A in liquid (gmole O3/m3); KG, overall mass-transfer coefficient based on gas-phase driving force (gmole/m2 · s · atm), pA partial pressure of O3 in bulk gas phase (atm); H, Henry€™s law constant for O3 between gas and liquid (m3 · atm/gmole); S, surface area for inter phase mass-transfer (m2).
e. What is the total transfer rate of O3, WA?
f. Is the mass-transfer process is gas film controlling, liquid film controlling, or neither? Comment on the relative contributions of the film mass-transfer coefficients and the equilibrium distribution relationship on the controlling mass-transfer resistance.
DistributionThe word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





