The contamination of water-saturated soils with toxic organic solvents is an important environmental problem. The organic solvents
Question:
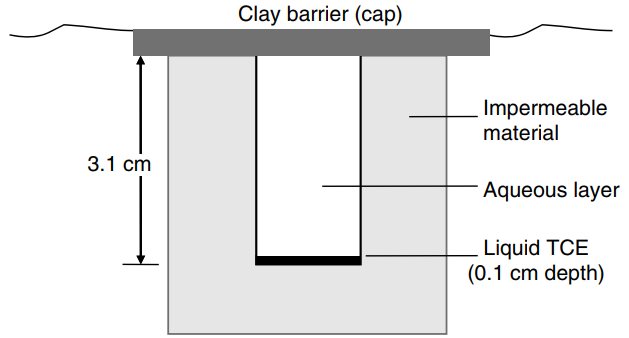 a. Initially, there is no TCE dissolved in the water. How long will it take for the TCE concentration in the water to reach 8.97 × 10-8gmole/cm3at a position of 0.3 cm from the organic-aqueous interface?
a. Initially, there is no TCE dissolved in the water. How long will it take for the TCE concentration in the water to reach 8.97 × 10-8gmole/cm3at a position of 0.3 cm from the organic-aqueous interface?b. What is the TCE concentration in the aqueous phase after infinite time (t †’ ˆž)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





