What is G at 25C for the reaction Sn 2+ (aq) + 2Hg 2+ (aq) Sn
Question:
What is ∆G° at 25°C for the reaction
Sn2+(aq) + 2Hg2+(aq) → Sn4+(aq) + Hg22+(aq)
For data, see Table 19.1.
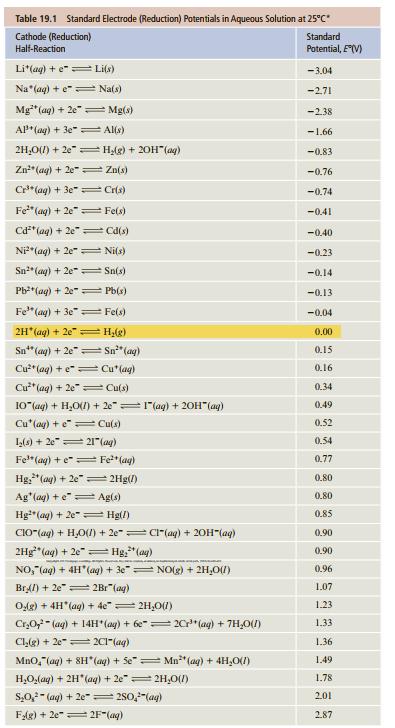
Transcribed Image Text:
Table 19.1 Standard Electrode (Reduction) Potentials in Aqueous Solution at 25°C Standard Cathode (Reduction) Half-Reaction Potential, E(V) Li*(ag) + e"= Li(s) - 3.04 Na"(aq) + e"= Na(s) -2.71 Mg"(ag) + 2e" = Mg(s) -2.38 AP* (ag) + 3e" = Al(s) -1.66 2H,O(1) + 2e"= Hlg) + 20H"(ag) -0.83 Zn"(ag) + 2e = Zn(s) -0.76 Cr*(aq) + 3e" Cris) -0.74 Fe" (ag) + 2e" Fe(s) -0.41 Cd*(ag) + 2e" = Cd(s) -0.40 Ni*(aq) + 2e" = Ni(s) -0.23 Sn"(ag) + 2e Sn(s) -0.14 Pb*(ag) + 2e" = Pb(s) -0.13 Fe" (ag) + 3e" = Fe(s) -0.04 2H (ag) + 2e H,(g) 0.00 Sn" (ag) + 2e"= Sn" (ag) 0.15 Cu**(aq) + e"= Cu*(ag) Cu**(ag) + 2e"= Cu(s) 10"(ag) + H,O) + 20"=1"(ag) + 20H" (ag) 0.16 0.34 0.49 Cu'(ag) + e= Cu(s) 0.52 0.54 L(s) + 2e"= 21"(ag) Fe"(ag) + e= Fe*(ag) 0.77 Hg;" (ag) + 2e =2Hg(/) 0.80 Ag'(ag) + e" Ag(s) 0.80 Hg"(ag) + 2e" = Hg(/) 0.85 CIO"(ag) + H,O() + 2e"= CI"(aq) + 20H"(ag) 2Hg" (aq) + 2e" = Hg," (ag) NO," (ag) + 4H (ag) + 3e"= NO(g) + 2H,01) 0.90 0.90 0.96 Brl) + 2e" 2Br"(ag) 1.07 Odg) + 4H"(ag) + 4e"= 2H,0(7) 1.23 Cr,O,- (ag) + 14H (ag) + 6e"= 2C"(ag) + 7H,0(1) 1.33 Cl,g) + 2e"= 2C"(ag) 1.36 Mno,"(aq) + SH (aq) + Se" Mn** (ag) + 4H,0(1) 1.49 H,O(ag) + 2H"(aq) + 2e"= 2H,0(1) 1.78 S,0,- (ag) + 2e-=280,"(ag) 2.01 FAg) + 2e" 2F"(ag) 287
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
The halfcell reactions the corresponding halfcell potentials and their sums are displayed ...View the full answer

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Table 10.16 gives data on the crime rate in 47 states in the United States for 1960. Try to develop a suitable model to explain the crime rate in relation to the 14 socioeconomic variables given in...
-
Attached are two data sets, one concerning Skittles and one about cereals. You may assume both samples provided were selected randomly and are representative of the population. Be sure to use the...
-
In Example 15.6, pure-component, liquid-phase adsorption data are used with the extended-Langmuir isotherm to predict a binary-solute data point. Use the following mixture data to obtain the best fit...
-
Find the volumes of the solids in Problems 4952 correct to the nearest unit. 4 cm -6 cm 8 cm
-
One of the most difficult tasks of developing and managing a global portfolio is assessing the risks of potential foreign investments. Duke University researcher C. R. Henry collaborated with two...
-
Assume the same information as in E14-4, except that Celine Dion Company uses the effective-interest method of amortization for bond premium or discount. Assume an effective yield of 9.7705%....
-
Pick any three of the sources of prospects discussed in the chapter and pick a product or service you like. Develop several ideas for how you would use each source to locate leads for the product or...
-
Calculate the profitability index for Problem 3. For Problem 4.
-
Carla Vista provides environmentally friendly lawn services for homeowners. Its operating costs are as follows. Depreciation $1,400 per month Advertising $350 per month Insurance $3,970 per month...
-
A system consisting of 1 kg of H2O undergoes a power cycle composed of the following processes: Process 1-2: Constant-pressure heating at 10 bar from saturated vapor Process 2-3: Constant-volume...
-
Define the faraday.
-
Consider the reducing agents Sn 2+ (aq), Cl 2 (g), and I (aq). Which is strongest? Which is weakest?
-
The following direct materials variance analysis was performed for Moore. Requirements 1. Record Moore's direct materials journal entries. Assume purchases were made on account. 2. Explain what...
-
A company's balance sheet shows: cash $30,000, accounts receivable $20,000, office equipment $54,000, and accounts payable $21,000. What is the amount of owner's equity?
-
Riemer, Incorporated has four departments. Information about these departments is listed below. Maintenance is a service department. If allocated maintenance cost i based on floor space occupied by...
-
Saved If an Invoice Indicates that interest at the rate of 0.68% per month will be charged on overdue amounts, what effective rate of Interest will be charged? (Round your final answer to 2 decimal...
-
Voluntary risk retention is a useful technique for handling risk for a large organisation and it can gain economic benefits if risk is managed appropriately. Voluntary risk retention is where the...
-
8.) Assume General Motors has a weighted average cost of capital of 9%. GM is considering investing in a new plant that will save the company $20 million over each of the first two years, and then...
-
Assume that the U.S. has a weak economy and that the Fed wants to correct this problem by adjusting the value of the dollar. The Fed is not worried about inflation. Assume that the Eurozone has a...
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
Formic acid, HCHO2, is a stronger acid than acetic acid, HC2H3O2. Which is the stronger base, formate ion, CHO2, or acetate ion, C2H3O2?
-
Rank the following solutions from most acidic to most basic (water molecules have been omitted for clarity).
-
You have solutions of NH3, HCl, NaOH, and HC2H3O2 (acetic acid), all with the same solute concentrations. Rank these solutions in order of pH, from the highest to the lowest.
-
Shawn Inc. proposed to install one Robotic Machine for his new project, an initial investment of CANVAS Technology will be $520,000 and the Robotic will be expected to generate net cash flows of at...
-
You anticipate the receipt of money in 200 days, which you will use to purchase stocks in a particular company. The stock is currently selling for $51 and will pay a $0.5 dividend in 50 days and...
-
1) Based on the stock chart for Michaels Companies Inc, what do you think the short and long-term growth potentials are for this company? (discuss the advantages/disadvantages) Link to the stock...

Study smarter with the SolutionInn App


