A hydrogen atom is placed in a uniform magnetic field B 0 = B 0 Z (the
Question:
A hydrogen atom is placed in a uniform magnetic field B0 = B0 Ẑ (the Hamiltonian can be written as in Equation 4.230). Use the Feynman– Hellman theorem (Problem 7.38) to show that

where the electron’s magnetic dipole moment (orbital plus spin) is
![]()
The mechanical angular momentum is defined in Equation 4.231.
Note: From Equation 7.114 it follows that the magnetic susceptibility of N atoms in a volume V and at 0 K (when they’re all in the ground state) is

where E0 is the ground-state energy. Although we derived Equation 7.114 for a hydrogen atom, the expression applies to multi-electron atoms as well—even when electron–electron interactions are included.
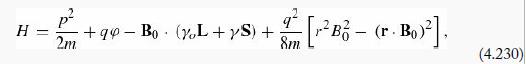
![]()
Step by Step Answer:

Introduction To Quantum Mechanics
ISBN: 9781107189638
3rd Edition
Authors: David J. Griffiths, Darrell F. Schroeter





