In the photoelectric effect, light can ionize an atom if its energy () exceeds the binding energy
Question:
In the photoelectric effect, light can ionize an atom if its energy (ћω) exceeds the binding energy of the electron. Consider the photoelectric effect for the ground state of hydrogen, where the electron is kicked out with momentum ћk. The initial state of the electron is Ψ0(r) (Equation 4.80) and its final state is
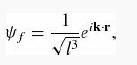
as in Example 11.2.
(a) For light polarized along the z direction, use Fermi’s Golden Rule to compute the rate at which electrons are ejected into the solid angle dΩ in the dipole approximation.
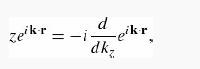
pull d/dkz outside the integral, and what remains is straightforward to compute.
(b) The photoelectric cross section is defined as
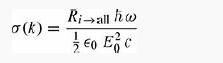
where the quantity in the numerator is the rate at which energy is absorbed ћω = ћ2k2/2m - E1 per photoelectron) and the quantity in the denominator is the intensity of the incident light. Integrate your result from (a) over all angles to obtain Ri → all, and compute the photoelectric cross section.
(c) Obtain a numerical value for the photoelectric cross section for ultraviolet light of wavelength 220A (n.b. this is the wavelength of the incident light, not the scattered electron). Express your answer in mega-barns (Mb = 10-22m2).

Step by Step Answer:

Introduction To Quantum Mechanics
ISBN: 9781107189638
3rd Edition
Authors: David J. Griffiths, Darrell F. Schroeter





