The density of copper is 8.96 g/cm 3 , and its atomic weight is 63.5 g/mole. (a)
Question:
The density of copper is 8.96 g/cm3, and its atomic weight is 63.5 g/mole.
(a) Calculate the Fermi energy for copper (Equation 5.54). Assume d = 1, and give your answer in electron volts.
(b) What is the corresponding electron velocity? Set EF = (1/2) mv2. Is it safe to assume the electrons in copper are nonrelativistic?
(c) At what temperature would the characteristic thermal energy (kB T , where kB is the Boltzmann constant and T is the Kelvin temperature) equal the Fermi energy, for copper? This is called the Fermi temperature, TF . As long as the actual temperature is substantially below the Fermi temperature, the material can be regarded as “cold,” with most of the electrons in the lowest accessible state. Since the melting point of copper is 1356 K, solid copper is always cold.
(d) Calculate the degeneracy pressure (Equation 5.57) of copper, in the electron gas model.
Equation 5.54

Equation 5.57
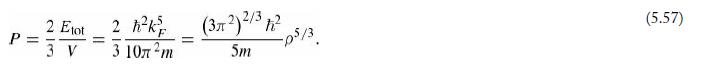
Step by Step Answer:

Introduction To Quantum Mechanics
ISBN: 9781107189638
3rd Edition
Authors: David J. Griffiths, Darrell F. Schroeter





