Fas ligand is a trimeric, extracellular protein that binds to its receptor, Fas, which is composed of
Question:
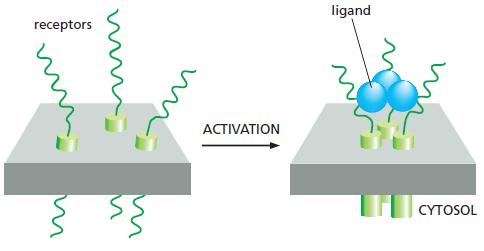
Fas ligand is a trimeric, extracellular protein that binds to its receptor, Fas, which is composed of three identical transmembrane subunits (Figure Q18–3). The binding of Fas ligand alters the conformation of Fas so that it binds an adaptor protein, which then recruits and activates caspase-8, triggering a caspase cascade that leads to cell death. In humans, the autoimmune lymphoproliferative syndrome (ALPS) is associated with dominant mutations in Fas that include point mutations and C terminal truncations. In individuals that are heterozygous for such mutations, lymphocytes do not die at their normal rate and accumulate in abnormally large numbers, causing a variety of clinical problems. In contrast to these patients, individuals that are heterozygous for mutations that eliminate Fas expression entirely have no clinical symptoms.
A. Assuming that the normal and dominant forms of Fas are expressed to the same level and bind Fas ligand equally, what fraction of Fas Fas ligand complexes on a lymphocyte from a heterozygous ALPS patient would be expected to be composed entirely of normal Fas subunits?
B. In an individual heterozygous for a mutation that eliminates Fas expression, what fraction of Fas–Fas ligand complexes would be expected to be composed entirely of normal Fas subunits?
C. Why are the Fas mutations that are associated with ALPS dominant, while those that eliminate expression of Fas are recessive?
Figure Q18-3
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter





