(a) Compute the heats of reaction for abstraction of a primary hydrogen and a secondary hydrogen from...
Question:
(a) Compute the heats of reaction for abstraction of a primary hydrogen and a secondary hydrogen from propane by a fluorine radical.
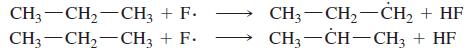
(b) How selective do you expect free-radical fluorination to be?
(c) What product distribution would you expect to obtain from the free-radical fluorination of propane?
Transcribed Image Text:
CH3-CH2-CH3 + F. CH3-CH,-CH3 + F. CH3 — CH, —Сн, + HF CH, — CH—СН, + HF
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
The overall in enthalpy change for the reaction is 157 KJmol b As we know that flooring is highly re...View the full answer

Answered By

NAROTTAM SHANDILYA
Heading my career into Teaching students for GATE/IES/PSU’s exam by seeing a great response from them.
1. GATE-2018, GATE-2019 & GATE-2020 Qualified.
2. Branch Topper in 7th SEM-B.TECH & 2nd Topper in 6th-SEM-B.TECH
Learning all management fundamentals and orienting myself to excel the management practices which are practically used in new start-ups and in Educational Institutes.
After gaining several years of experience and expertise to grasp the knowledge of Thermal Machineries, Manufacturing Methods, Design of refrigeration Ducts, Design of Structures and implementing this knowledge with IoT to experience designing and implementing solutions, hence looking for a similar role and position in a progressive organization to gain such an experience and knowledge to evenly spread such knowledge to the seekers and students.
Planning to put my future 5 years of learning into use to help the students in becoming more strategic towards their goals by identifying opportunities & deploying new methodology of teaching.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The observed relative reactivities of primary, secondary, and tertiary alcohols with a hydrogen halide are 3o> 2o> 1o.If secondary alcohols underwent an reaction rather than an reaction with a...
-
a. Propose a mechanism for the following reaction: b. Is the initially formed carbocation primary, secondary, or tertiary? c. Is the rearranged carbocation primary, secondary, or tertiary? d. Why...
-
Figure 3-9 compares the reactions of CI with the primary and secondary hydrogens of propane. (a) Draw a similar diagram comparing the reactions of Br with the primary and secondary hydrogens of...
-
Find the area between the parabolas y = 2x 2 + 1 and y = x 2 + 5.
-
Write the Henderson-Hasselbalch equation for a solution of formic acid. Calculate the quotient [HCO-2 ]/[HCO2H] at (a) pH 3.000; (b) pH 3.744; (c) pH 4.000.
-
Why does a firm incur fixed costs over the short run when its output is zero, and why do fixed costs not change as the level of output changes? Why do a firms short-run total variable costs increase...
-
Until recently, Seth worked for the Seaside Cruise Ship Line Corporation (Seaside) as an engineer. Seaside is a U.S. company based in Florida. For the past two years, Seth has been assigned to one of...
-
You are the vice president of International InfoXchange, headquartered in Chicago. All shareholders of the firm live in the United States. Earlier this month, you obtained a loan of 5 million...
-
Marshall's concept of external economies and diseconomies refers to: a) Changes in output resulting from changes in input levels b) The effects of production on the environment c) The benefits or...
-
In a survey of 529 travelers, 386 said that location was very important and 323 said that room quality was very important in choosing a hotel. a. Construct a 95% confidence interval estimate for the...
-
(a) When n-heptane burns in a gasoline engine, the combustion process takes place too quickly. The explosive detonation makes a noise called knocking. When 2,2,4-trimethylpentane (isooctane) is...
-
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these...
-
Several years ago in its annual report, Philip Morris Companies, a major manufacturer of tobacco and food products, included footnote 16, which was almost five pages long. It consisted of a number of...
-
First, be sure to read R. V. Vargas's article, "Avoiding mistakes during the team acquisition: find the right people to the right function using MBTI" Second, even if you've done this before,...
-
For this discussion, think about how communication plans and methods are affected when some project team members or stakeholders are in a different location. Then, make a post that answers the...
-
on Solve the following equations by finding Suitable vdm + n (n+v) dv=o integerating factor
-
Use this lecture outline/guide to add notes from your reading under each topic. Include references to examples, exercises and problems that illustrate the concept. How is a predetermined overhead...
-
A steel rod, which is free to move, has a length of 200 mm and diameter of 20 mm at a temperature of 15C. If the rod is heated uniformly to 115C, determine the length and the diameter of this rod to...
-
Two objects carry initial charges that are q1 and q2, respectively, where |q2| > |q1|. They are located 0.200 m apart and behave like point charges. They attract each other with a force that has a...
-
Halley's comet travels in an ellipti- cal orbit with a = 17.95 and b = 4.44 and passes by Earth roughly every 76 years. Note that each unit represents one astronomical unit, or 93 million miles. The...
-
Calculate the frequency of Blue light with = 4800 A
-
Five isomeric alkenes A-E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm-1): Compound A: 912 (s), 994...
-
From the information in Table 12.3, predict the appearance of the molecular ion peak(s) in the mass spectrum of chloromethane. (Assume that the molecular ion is the base peak.) Table 12.3 TABLE 12.3...
-
How crucial is it to understand the many sorts of speech contexts on a daily basis? Give one specific example. Here are the types of speech context: 1. Public Communication 2. Small Group...
-
Enmebaragesi of Kish, Inc. has sales of $282,000, cost of goods sold of $206,000, depreciation of $4,200, and interest expense of $22,000. The tax rate is 30.8 percent. What is the times interest...
-
Inkishush and Company has a book value per share of $10.35, earnings per share of $2.23, and a price-earnings ratio of 19.4. What is the market-to-book ratio?

Study smarter with the SolutionInn App


