A new graduate student was studying the insecticidal properties of a series of polycyclic epoxides. He epoxidized
Question:
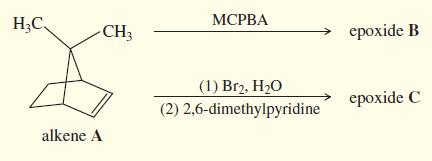
A new graduate student was studying the insecticidal properties of a series of polycyclic epoxides. He epoxidized alkene A using two different methods. First he used MCPBA, which gave an excellent yield of an epoxide that he labeled B. Then he treated alkene A with bromine water to form the bromohydrin, followed by 2,6-dimethylpyridine (see page 647) to form an epoxide in fair yield. To his surprise, the second method produced an epoxide (C) with different physical and chemical properties from the first. In particular, C reacts with strong nucleophiles much faster than B. Propose structures for B and C, and propose mechanisms to show why different products are formed. Explain why C reacts so much faster with strong nucleophiles.
Step by Step Answer:






