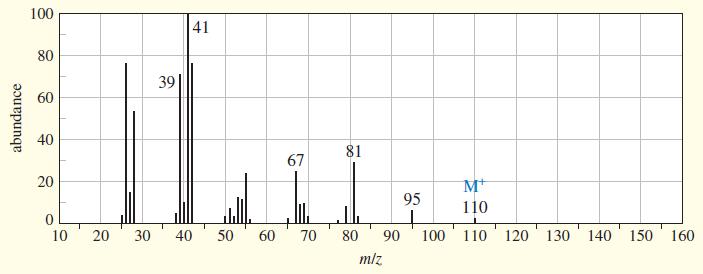
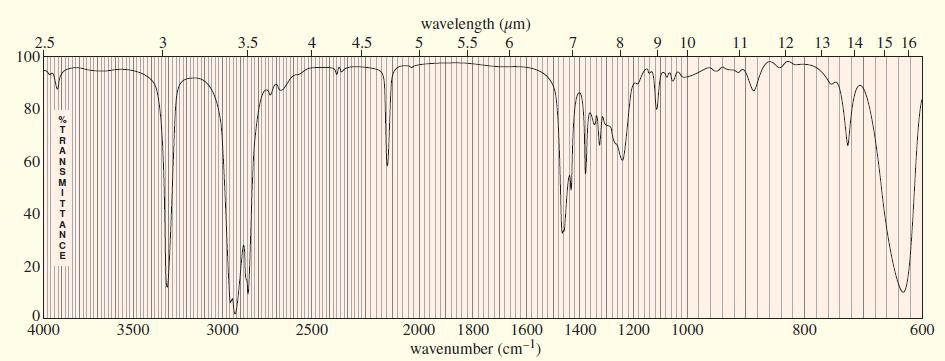
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown. (a) Use the mass spectrum
Question:
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
(a) Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
(b) Use the IR spectrum to determine the functional group(s), if any.
(c) Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
(d) Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum. Explain why the base peak is so strong.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





