Chapter 9 covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to
Question:
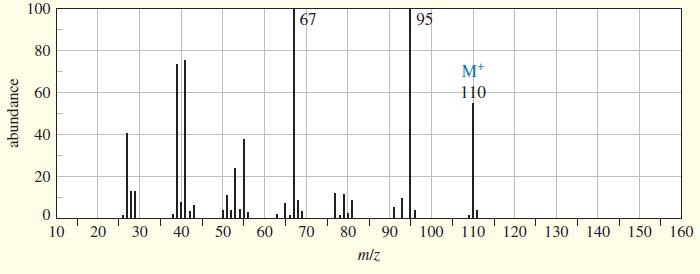
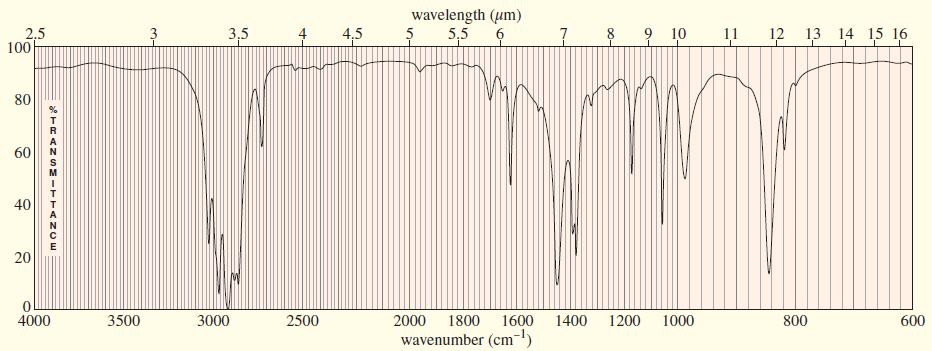
Chapter 9 covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to convert trans-2,5-dimethylhex-3-ene to 2,5-dimethylhex-3-yne by adding bromine across the double bond, then doing a double elimination. The infrared and mass spectra of the major product are shown here.
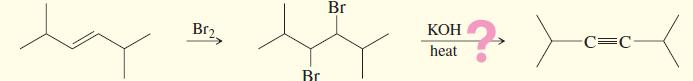
(a) Do the spectra confirm the right product? If not, what is it?
(b) Explain the important peaks in the IR spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





