The C=O double bond has a dipole moment of about 2.4 D and a bond length of
Question:
The C=O double bond has a dipole moment of about 2.4 D and a bond length of about 1.23 Å.
(a) Calculate the amount of charge separation in this bond.
(b) Use this information to evaluate the relative importance of the following two resonance contributors:
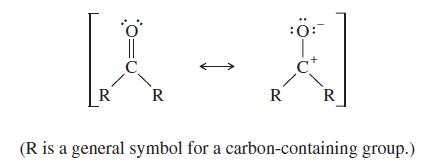
Transcribed Image Text:
:ö:- R R R R (R is a general symbol for a carbon-containing group.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)

Answered By

CHINKY GANGWAR
I had completed my master's in chemistry from university of Lucknow and currently pursuing PhD from the same University. I had worked in bsnv pg college lucknow for 7months as a part time lecturer in department of chemistry.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The iodine mono bromide molecule, IBr, has a bond length of 2.49 and a dipole moment of 1.21 D. (a) Which atom of the molecule is expected to have a negative charge? Explain. (b) Calculate the...
-
The PF3 molecule has a dipole moment of 1.03 D, but BF3 has a dipole moment of zero. How can you explain the difference?
-
Two molecules, each with the general formula AX3, have different dipole moments. Molecule Y has a dipole moment of zero, whereas molecule Z has a nonzero dipole moment. From this information, what...
-
In Exercises simplify the ratio of factorials. (n + 1)! n!
-
Write the chemical reaction whose equilibrium constant is (a) Ka for benzoic acid, C6H5CO2H (b) Kb for benzoate ion, C6H5CO-2 (c) Kb for aniline, C6H5NH2 (d) Ka for anilinium ion, C6H5NH+3
-
What is the result of compiling and executing the following class? A. The code does not compile. B. 4 C. 5 D. 10 E. 20 public class RollerSkates { static int wheels = 1; int tracks = 5; public static...
-
The plaintiff, Dennis Rubel, was permanently injured while working at Lowes Home Center Inc., and hired Michael Dzienny to represent him in a personal injury lawsuit. When discussing a settlement...
-
Selected accounts from the general ledger of Tucker Consulting Services follow. Record the general journal entries that would be made to record the following transactions. Be sure to include dates...
-
Discuss the following topic(s) in the forum and submit proof of your participation in the online discussions: 1) Professional certification and the practising accountants 2) Cost data and managerial...
-
A survey conducted by the National Endowment for the Arts asked, Have you read a book within the last year? What response bias might arise from this question?
-
Give the relationship between the following pairs of structures. The possible relationships are: same compound cis-trans isomers constitutional isomers (structural isomers) not isomers (different...
-
The N-F bond is more polar than the N-H bond, but NF 3 has a smaller dipole moment than NH 3 . Explain this curious result. NH 3 .... NF 3 = 1.5D = 0.2D
-
In the early 1600s, Jean Baptista van Helmont investigated how plants acquire new mass as they grow. He weighed a willow shoot and planted it into soil that he had also weighed. After 5 years of...
-
Why might one prefer to use the logarithmic differentiation for finding derivatives of functions that have another function as an exponent?
-
Shea is pricing materials (wood, wire, pipe, etc.) for new home construction on a "per unit" basis. Inflation on materials has been running at 16.0% for the past 3 years and is expected to remain at...
-
The following are the financial statements of Nosker Company. NOSKER COMPANY Comparative Balance Sheets December 31 Assets 2020 2019 Cash $34,000 $19,100 Accounts receivable 32,900 18,750 Inventory...
-
Carole owns 100% of Carole Inc, and her spouse Carl owns 100% of Carl Inc (both CCPCs). Carole is considering purchasing 30% of the common shares of Carl Inc. Explain to Carole how this transaction...
-
Rest Up Mattresses had a purchase of $ 2 0 , 0 0 0 , freight charges of $ 2 5 0 , a purchase return of $ 9 , 7 5 0 , and a purchase discount of $ 2 , 2 2 5 . What is the impact on Inventory?
-
A spring with an unstrained length of 0.074 m and a spring constant of 2.4 N/m hangs vertically downward from the ceiling. A uniform electric field directed vertically upward fills the region...
-
Use a calculator to evaluate the expression. Round your result to the nearest thousandth. V (32 + #)
-
Explain why all attempts to isolate trimethyloxonium iodide lead instead to methl iodide and dimethl ether.
-
Give the structure of an intramoleczlar substitution product and an intermolecular substitution product that might be obtained from 4-bromo-l-butanol on treatment with one equivalent of NaOH. Which...
-
Give the structure of an intramoleczlar substitution product and an intermolecular substitution product that might be obtained from 4-bromo-l-butanol on treatment with one equivalent of NaOH. Which...
-
Sandy Bank, Incorporated, makes one model of wooden canoe. Partial information is given below. Required: 1. Complete the following table. 2. Suppose Sandy Bank sells its canoes for $510 each....
-
FIFO and LIFO costs under perpetual inventory system The following units of an item were available for sale during the year: 29 units at $44 Beginning inventory Sale 26 units at $64 First purchase 15...
-
Pie Corporation acquired 75 percent of Slice Company's ownership on January 1, 20X8, for $93,000. At that date, the fair value of the noncontrolling interest was $31,000. The book value of Slice's...

Study smarter with the SolutionInn App


