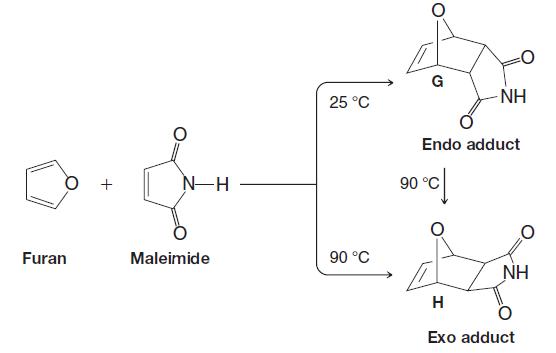
When furan and maleimide undergo a DielsAlder reaction at 25 C, the major product is the endo
Question:
When furan and maleimide undergo a Diels–Alder reaction at 25 °C, the major product is the endo adduct G. When the reaction is carried out at 90 °C, however, the major product is the exo isomer H. The endo adduct isomerizes to the exo adduct when it is heated to 90 °C. Propose an explanation that will account for these results.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry
ISBN: 978-1118133576
11th edition
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
Question Posted:





