Identify the most acidic proton in each of the following compounds and explain your choice: (a) (b)
Question:
(a)
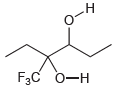
(b)
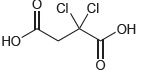
Transcribed Image Text:
0-H F3C 0-H CI. CI ОН но
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a The highlighted proton is more acidic When this location is deprotonated the conjugate ba...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton in each compound, and suggest a reason for the trend in...
-
Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton in each compound, and suggest a reason for the trend in...
-
For each compound below, identify the most acidic proton in the compound: (a) (b) (c) (d) (e) (f) (g) (h) -NH2 -
-
In Exercises, analyze and sketch a graph of the function. Label any intercepts, relative extrema, points of inflection, and asymptotes. Use a graphing utility to verify your results. f(x)=x16 - x
-
Luke Skywalker College has chosen to report as a public university reporting as a special-purpose entity engaged. Deferred Revenues were reported as of July 1, 2014 in the amount of $5,000,000....
-
Periodic versus Perpetual Entries Chippewas Company sells one product. Presented below is information for January for Chippewas Company. Jan. 1 Inventory 100 units at $6 each 4 Sale 80 units at $8...
-
A local theater company was seeking unpaid interns to be involved in their summer theater productions. The company hired several students from Better Business University to fill these slots. At the...
-
LIFO Effect The following example was provided to encourage the use of the LIFO method. In a nutshell, LIFO subtracts inflation from inventory costs, deducts it from taxable income, and records it in...
-
The price of a car you want is $39,000 today. Its price is expected to increase by $1000 each year. You now have $23,500 in an investment account, which is earning 11% per year. How many years will...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
In the following compound two protons are clearly identified. Determine which of the two is more acidic. After comparing the conjugate bases, you should get stuck on the following question: Is it...
-
For each pair of compounds below, identify which compound is more acidic and explain your choice: (a)
-
What is the purpose of employee knowledge networks?
-
A small country can import a good at a world price of 5 per unit. The domestic supply curve of the good is S = 10 + 10P. The demand curve is D = 600 - 5P. In addition, each unit of production yields...
-
If all colleges are private and the market for education is competitive, calculate the number of students, the tuition, and the deadweight loss. The marginal cost of educating a student is a constant...
-
In December 2019, Mr. Joe Jacobs cashed qualified Series EE U.S. Savings Bonds, which he had purchased in January 2012. The proceeds were used for his son's college education. All of the following...
-
All of the following fringe benefits can be excluded from the employees income except: a. Transportation up to $265 per month for combined commuter highway vehicle transportation and transit passes,...
-
If the government provides public colleges, what tuition will achieve the efficient number of students? How much will taxpayers have to pay? The marginal cost is a constant $6,000 per student per...
-
McEwan Industries sells on terms of 3/10, net 30. Total sales for the year are $1,921,000; 40% of the customers pay on the 10th day and take discounts,while the other 60% pay, on average, 70 days...
-
According to a recent survey, 40% of millennials (those born in the 1980s or 1990s) view themselves more as spenders than savers. The survey also reveals that 75% of millennials view social...
-
Suggest an experiment using an isotopically labeled alcohol that would prove that the formation of an alkyl sulfonate does not cause cleavage at the C-O bond of the alcohol.
-
An exception to what we have just said has to do with syntheses of unsymmetrical ethers in which one alkyl group is a tert-butyl group and the other group is primary. For example, this synthesis can...
-
(a) Outline two methods for preparing isopropyl methyl ether by a Williamson ether synthesis. (b) One method gives a much better yield of the ether than the other. Explain which is the better method...
-
Terminal value is when a project's cash flows are arbitrarily truncated. Question 15 options: True False
-
Kevin and his best buddy, Aaron, own a sporting goods store in Aspen, Colorado. They each make $ 5 , 1 0 0 in personal monthly income by selling ski gear, clothes, hiking accessories, and other...
-
Please answer there questions in excel and show how you did it for example fv=b3,b3/b7, something like that show and make sure ik what problem you are doing like this answer is for the first one and...

Study smarter with the SolutionInn App


