Malonic acid has two acidic protons: The pKa of the first proton (pK 1 ) is measured
Question:
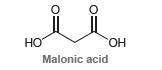
The pKa of the first proton (pK1) is measured to be 2.8, while the pKa of the second proton (pK2) is measured to be 5.7.
(a) Explain why the first proton is more acidic than acetic acid (pKa = 4.76).
(b) Explain why the second proton is less acidic than acetic acid.
(c) Draw the form of malonic acid that is expected to predominate at physiological pH.
(d) For succinic acid (HO2CCH2CH2CO2H), pK1 = 4.2 (which is higher than pK1 for malonic acid) and pK2 = 5.6 (which is lower than pK2 for malonic acid). In other words, the difference between pK1 and pK2 is not as large for succinic acid as it is for malonic acid. Explain this observation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





