Part 1 a. Both NaCl and MgCl 2 are soluble ionic compounds. Write the balanced chemical equations
Question:
Part 1 a. Both NaCl and MgCl2 are soluble ionic compounds. Write the balanced chemical equations for these two substances dissolving in water.
b. Consider the pictures below. These pictures represent 1.0-L solutions of 1.0 M NaCl(aq) and 1.0 M MgCl2(aq). The representations of the ions in solution are the correct relative amounts. Water molecules have been omitted for clarity. Correctly label each of the beakers, provide a key to help identify the ions, and give a brief explanation of how you made your assignments.
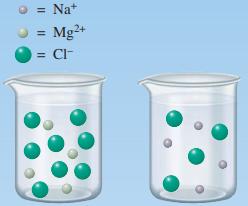
Keeping in mind that the pictures represent the relative amounts of ions in the solution and that the numerical information about these solutions is presented above, answer the following questions c through f.
c. How many moles of NaCl and MgCl2 are in each beaker?
d. How many moles of chloride ions are in each beaker? How did you arrive at this answer?
e. What is the concentration of chloride ions in each beaker? Without using mathematical equations, briefly explain how you obtained your answer.
f. Explain how it is that the concentrations of chloride ions in these beakers are different even though the concentrations of each substance (compound) are the same.
Part 2: Say you were to dump out half of the MgCl2 solution from the beaker above.
a. What would be the concentration of the MgCl2(aq) ion and of the chloride ions in the remaining solution?
b. How many moles of the MgCl2 and of the chloride ions would remain in the beaker?
c. Explain why the concentration of MgCl2(aq) would not change, whereas the number of moles of MgCl2 would change when solution was removed from the beaker. As part of your answer, you are encouraged to use pictures.
Part 3: Consider the beaker containing 1.0 L of the 1.0 M NaCl(aq) solution. You now add 1.0 L of water to this beaker.
a. What is the concentration of this NaCl(aq) solution?
b. How many moles of NaCl are present in the 2.0 L of NaCl(aq) solution?
c. Explain why the concentration of NaCl(aq) does change with the addition of water, whereas the number of moles does not change. Here again, you are encouraged to use pictures to help answer the question.
Step by Step Answer:






