The presence of additional nitro groups can have an impact on the temperature at which a nucleophilic
Question:
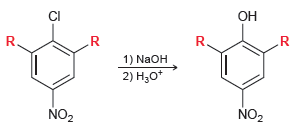
When both R groups are hydrogen atoms, the reaction readily occurs at 130°C. When one of the R groups is a nitro group, the reaction readily occurs at 100°C. When both R groups are nitro groups, the reaction readily occurs at 35°C.
(a) Provide an explanation that justifies the lower temperature requirement with additional nitro groups.
(b) If a fourth nitro group is placed on the ring, would you expect the temperature requirement to be further lowered? Explain your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





