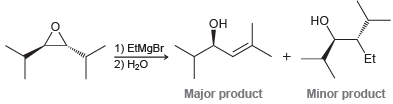
The S N 2 reaction between a Grignard reagent and an epoxide works reasonably well when the
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





