We have seen that NaH is a strong base but a weak nucleophile. In contrast, lithium aluminum
Question:
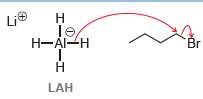
In this case, LAH functions as a delivery agent of a nucleophilic hydride ion. We will see this reagent in many upcoming chapters. Explain why LAH is capable of functioning as a strong nucleophile while NaH is not.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





