When 3,3-dichloropentane is treated with excess sodium amide in liquid ammonia, the initial product is 2-pentyne: However,
Question:
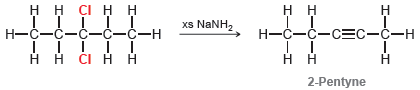
However, under these conditions, this internal alkyne quickly isomerizes to form a terminal alkyne that is subsequently deprotonated to form an alkynide ion:
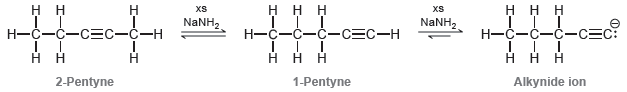
The isomerization process is believed to occur via a mechanism with the following four steps:
(1) Deprotonate
(2) Protonate
(3) Deprotonate
(4) Protonate.
Using these four steps as a guide, try to draw the mechanism for isomerization using resonance structures whenever possible. Explain why the equilibrium favors formation of the terminal alkyne.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





