When ethylene oxide is treated with a strong nucleophile, the epoxide ring is opened to form an
Question:
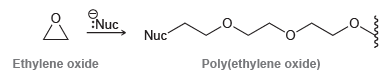
The resulting polymer is called poly(ethylene oxide) or poly(ethylene glycol). It is sold under the trade name Carbowax and is as an adhesive and a thickening agent.
(a) Draw a mechanism showing the formation of a segment of poly(ethylene oxide).
(b) Ethylene oxide can also be polymerized when treated with an acid. Draw the mechanism of formation of a segment of poly(ethylene oxide) under acidic conditions.
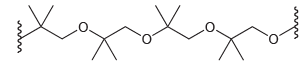
(c) Identify the monomer you would use to prepare the following polymer:
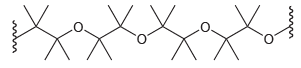
(d) Determine whether you would use basic conditions or acidic conditions to prepare the following polymer. Explain your choice.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





