A dilute solution of a weak (1,1)-electrolyte contains both neutral ion pairs and ions in equilibrium (AB
Question:
A dilute solution of a weak (1,1)-electrolyte contains both neutral ion pairs and ions in equilibrium (AB ⇌ A+ +B−). Prove that molar conductivities are related to the degree of ionization by the equations:
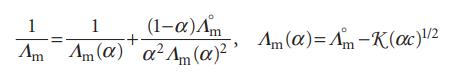
where Λm° is the molar conductivity at infinite dilution and K is the constant in Kohlrausch’s law.
Transcribed Image Text:
1 1 (1-α).m Am Am(α)'a'Am(α) + Am(a)= Am-K(ac)1/2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Thorough calculation The molar conductivity of an electrolyte is gi...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
A few of the guidelines are related to the need to understand the reason for the project in the first place. Which guidelines would you place in this category? Why is this so crucial?
-
A dilute solution of potassium permanganate in water at 25C was prepared. The solution was in a horizontal tube of length 10 cm, and at first there was a linear gradation of intensity of the purple...
-
The personality characteristics of business leaders (e.g., CEOs) are related to the operations of the businesses that they lead (Oreg & Berson, 2018). Traits like openness to experience are related...
-
Mr. Lawrence had been the manager at Pleasure Sdn Bhd (PSB) since 1 February 2016. His employment was terminated on 30 April 2021 due to disputes with the directors of PSB. After many appeals,...
-
There are many cases of serious wage and hour violations by employers, particularly in the retail sector. These cases feature employers failing to provide breaks, pressuring workers to underreport...
-
A MPV has seven passenger seats one in the front, and three in each of the other two rows. a. In how many ways can all 8 seats be filled from a party of 12 people, assuming that they can all drive?...
-
The Korvette concept was started and run by one person and his group of friends. How could its failure have been avoided? Was the problem one of strategy (overexpansion), or was it organizational?...
-
The Raab Company is expanding its production facilities to include a new product line, a sporty automotive tire rim. Tire rims can now be produced with little labor cost using new computerized...
-
1 Problem 4 - A firm's optimal output choice (10 points) Consider a price taking firm, where the market price for the output is given by P. The firm's output choice is denote by Q. The firm has a...
-
Dani deposited $20,000 into an account that compounds interest monthly at a rate of 1.26%. His plan is to use the account to make direct withdrawals each month of $800 to pay his rent. How many...
-
Calculate the relation between x 2 1/2 and x 4 1/4 for diffusing particles at a time t if they have a diffusion constant D.
-
What are the drift speeds of Li + , Na + , and K + in water when a potential difference of 100V is applied across a 5.00 cm conductivity cell? How long would it take an ion to move from one electrode...
-
Make a complete graph of the following functions. If an interval is not specified, graph the function on its domain. Use a graphing utility to check your work. |f(x) = x + tan x on
-
What is the true currency of the financial Market? A) Ether B) Blood C) Probability D) Dollars E) Satoshis
-
Find the annual simple discount rate equivalent to an annual simple interest rate of 7.2% over a term of 6 years. Round your answer to the nearest tenth of a percent.
-
When an aircraft mechanic or repairman adds or removes any item on the equipment list, he or she must change the_____to indicate the new empty weight and EWCG, and the equipment list is revised to...
-
is Cloning is currently only possible for livestock. Group of answer choices True False
-
When buying a car, it's always best to finance car through the dealership since they have access to loans with the lowest interest rates. Group of answer choices True False
-
a) Let a X. Prove that if xn = a for every n N, then xn converges. What does it converge to? b) Let X = R with the discrete metric. Prove that xn a as n if and only if xn = a for large
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
Is (1, 2) = 1s(1) (1)1s(2) (2) + 1s(2) (2)1s(1) (1) an eigenfunction of the operator S z ? If so, what is its eigenvalue M S ?
-
Calculate the angles that a spin angular momentum vector for an individual electron can make with the z axis.
-
In this problem we represent the spin eigenfunctions and operators as vectors and matrices. a. The spin eigenfunctions are often represented as the column vectors Show that α and...
-
To verify that the transition matrices P and P are inverse to one another, we need to show PP=1 P'P=I, Where I is the 33 identity matrix, 1 0 0 I=0 10 001 -21 -2 0 0 1 PP 2 1 4 100 2/3 1/3 2/3 -1/6...
-
4. (a) For what values of a is F = (x + yz)i+a(y+2zx)] + (xy + z)k a conservative vector field? For this value of a, find a potential p such that F= Vip. (b) A particle is moved from the origin (0,...
-
For each function, state whether it is (i) bounded and piecewise monotonic; (ii) piecewise con- tinuous; (iii) integrable on the domain given. No justification needed. (a) f [1,1] R, f(x) = |x| : (b)...

Study smarter with the SolutionInn App


