A methanol-synthesis reactor is fed with a gas stream at 220C consisting of 5.0 mole% methane, 25.0%
Question:
A methanol-synthesis reactor is fed with a gas stream at 220°C consisting of 5.0 mole% methane, 25.0% CO, 5.0% CO2, and the remainder hydrogen. The reactor and feed stream are at 7.5 MPa. The primary reaction occurring in the reactor and its associated equilibrium constant are
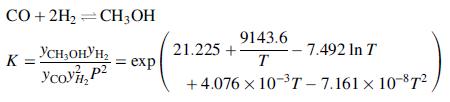
where T is in kelvins. The product stream may be assumed to reach equilibrium at 250°C.
(a) Determine the composition (mole fractions) of the product stream and the percentage conversions of CO and H2.
(b) Neglecting the effect of pressure on enthalpies, estimate the amount of heat (kJ/mol feed gas) that must be added to or removed from (state which) the reactor.
(c) Calculate the extent of reaction and heat removal rate (kJ/mol feed) for reactor temperatures between 200°C and 400°C in 50°C increments. Use these results to obtain an estimate of the adiabatic reaction temperature.
(d) Determine the effect of pressure on the reaction by evaluating extent of conversion and rate of heat transfer at 1 MPa and 15 MPa.
(e) Considering the results of your calculations in Parts (c) and (d), propose an explanation for selection of the initial reaction conditions of 250°C and 7.5 MPa.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





