Between 0°C and 100°C, the heat capacity of Hg(l) is given by Calculate ÎH and ÎS if
Question:
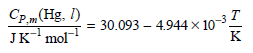
Calculate ΔH and ΔS if 2.25 moles of Hg(l) is raised in temperature from 0.00° to 88.0°C at constant P.
Transcribed Image Text:
Cp.„(Hg. I) 30.093 – 4.944 × 10-32 JK' mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
36115 AH n Cpmd TK 27315 225 mol 30093 ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 . Assume that the heat capacity of Pb(s) is given by The melting point is 327.4C and the heat of fusion under these conditions is...
-
The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by β = 0.3530 à 10 4 K 1 and V m = 22.6 cm 3 mol+. Calculate ÎS m for...
-
A 1.75 mole sample of an ideal gas is compressed isothermally from 62.0 L to 19.0 L using a constant external pressure of 2.80 atm. Calculate q, w, U, and H.
-
A 200 g mass attached to a horizontal spring oscillates at a frequency of 1.5 Hz. At one instant, the mass is at x = 70 mm and has vx = -0.2 m/s. Determine (a) The period (b) The amplitude (c) The...
-
Silva Piping Company produces PVC piping in two processing departments-Fabrication and Packaging. Transactions for the month of July are shown as follows. 1. Direct materials totaling $15,000 are...
-
Determine the structure of this compound from its IR and 13C-NMR spectra, its formula isC7H16O2: C,H1,0, 80- 60- %T 40 20- 0- 500 1000 1500 2000 2500 3500 3000 4000 Wavenumber (cm) C,H1602 All CH's...
-
Explain the term private placement. What are its advantages and disadvantages within the financial markets?
-
Sunflower Architects incorporated as licensed architects on April 1, 2010. During the first month of the operation of the business, these events and transactions occurred: Apr. 1 Stockholders...
-
A student addresses a bully mocking another student, " what you're doing isn't even clever or funny, everyone has a right ti express who they are.. you know like we say every morning.. liberty and...
-
Lots of data are available to retailers to make good decisionsloyalty programs, Web analytics, and point-of-sale data. However, there is a big gap between having data and being able to leverage them...
-
The initiation step for radical addition of HBr is highly endothermic: (a) Explain how this step can be thermodynamically favorable at high temperature even though it is endothermic. (b) Explain why...
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
The moist unit weight of a soil is 19.2 kN/m3. Given that Gs = 2.69 and w = 9.8%, determine: a. Void ratio b. Dry unit weight c. Degree of saturation
-
For the sequence \[x(n)= \begin{cases}1, & 0 \leq n \leq 1 \\ 0, & \text { otherwise }\end{cases}\] compute \(y(n)=x(n) * x(n) * x(n) * x(n)\). Check your results using the MATLAB function conv.
-
Home Innovations is evaluating a new product design. The estimated receipts and disbursements associated with the new product are shown below. MARR is 10 percent/year. a. What is the discounted...
-
Which of the following is a liability? (A) Machinery (B) Accounts payable for goods (C) Motor vehicles (D) Cash at bank
-
Which of the following should not be called 'Sales'? (A) Office fixtures sold (B) Goods sold on time (C) Goods sold for cash (D) Sale of item previously included in 'Purchases'
-
Which of the following best describes the meaning of 'Purchases'? (A) Items bought (B) Goods bought on time (C) Goods bought for resale (D) Goods paid for
-
Find the transfer function H(s) = V o V i of the circuit shown in Fig. 14.71 . V. ll V, (+)
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Repeat the calculation in Problem 11.4but plot the probability densities of the two orbitals. Then form the difference density, the difference between 2 and | 2a + 2b|
-
Imagine a small electron-sensitive probe of volume 1.00 pm3 inserted into an H+2 molecule-ion in its ground state. Calculate the probability that it will register the presence of an electron at the...
-
The same data as in Problem 11.8 may be used to calculate the molecular potential energy curve for the antibonding orbital, which is given by Plot the curve
-
Describe, in steps, at least one strategy used to attack embedded operating systems. Describe, in steps, at least one strategy used to defend against the chosen attack.
-
how does a hypercompetetice environment challenge those in an industry
-
Describe how managers can reduce unnecessary costs, including real - world examples of how it has been done

Study smarter with the SolutionInn App


