Biodiesel fuela sustainable alternative to petroleum diesel as a transportation fuelis produced via the transesterification of triglyceride
Question:
Biodiesel fuel—a sustainable alternative to petroleum diesel as a transportation fuel—is produced via the transesterification of triglyceride molecules derived from vegetable oils or animal fats. For every 9 kg of biodiesel produced in this process, 1 kg of glycerol, C3H8O3, is produced as a byproduct. Finding a market for the glycerol is important for biodiesel manufacturing to be economically viable.
A process for converting glycerol to the industrially important specialty chemical intermediates acrolein, C3H4O, and hydroxyacetone (acetol), C3H6O2, has been proposed.13
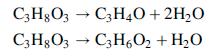
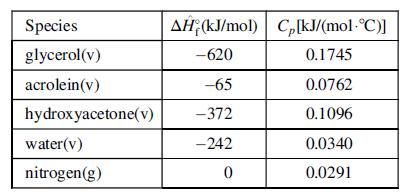
The reactions take place in the vapor phase at 325°C in a fixed bed reactor over an acid catalyst. The feed to the reactor is a vapor stream at 325°C containing 25 mol% glycerol, 25% water, and the balance nitrogen. All of the glycerol is consumed in the reactor, and the product stream contains acrolein and hydroxyacetone in a 9:1 mole ratio. Data for the process species are shown below.
(a) Assume a basis of 100 mol fed to the reactor, and draw and completely label a flowchart. Carry out a degree-of-freedom analysis assuming that you will use extents of reaction for the material balances. Then calculate the molar amounts of all product species.
(b) Calculate the total heat added or removed from the reactor (state which it is), using the constant heat capacities given in the above table.
(c) Assuming this process is implemented along with biodiesel production, how would you determine whether the biodiesel is an economically viable alternative to petroleum diesel?
(d) If you do a degree-of-freedom analysis based on atomic species balances, you are likely to count one more equation than you have unknowns, and yet you know the system has zero degrees of freedom. Guess what the problem is, and then prove it.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





