Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using
Question:
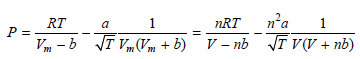
The Redlich€“Kwong parameters a and b for benzene are 452.0 bar dm6 molˆ’2 K1/2 and 0.08271dm3 molˆ’1, respectively. Is the attractive or repulsive portion of the potential dominant under these conditions?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





