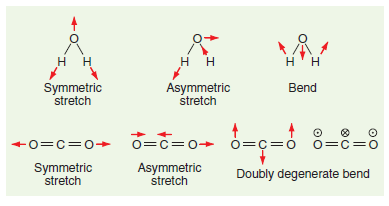
Calculating the motion of individual atoms in the vibrational modes of molecules (called normal modes) is an
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





