Citric acid (C 6 H 8 O 7 ) is used in the preparation of many foods,
Question:
Citric acid (C6H8O7) is used in the preparation of many foods, pharmaceuticals, soft drinks, and personal-care products. Although it can be recovered by concentration and crystallization from citrus juices, especially lemons, modern commercial production involves synthesis by fermentation of molasses or other carbohydrates such as glucose or fructose by the fungus Aspergillus niger (A. niger). The process involves addition of the fungus to a fermenter along with glucose, nutrients, water, and air that is bubbled through the fermentation broth. After the desired conversion, the resulting liquor is processed first by filtration of the cell mass and other solids from the liquid and then recovery and purification of the citric acid by crystallization.
As part of the evaluation of a proposed continuous fermentation process, you have been asked to estimate the heating or cooling requirement associated with a fermenter that is to produce 10.0 kg of citric acid per hour. Feed to the unit includes (1) an aqueous solution that is 20.0 wt% glucose (C6H12O6), 0.4% ammonia, and the remainder water; and (2) air at 1.2 atm, saturated with water, providing a molar flow rate of oxygen twice that of the glucose. Leaving the fermenter are (3) a gas stream at 1 atm containing N2, unreacted O2, and CO2 formed by fermentation, and saturated with water, and (4) a liquid stream containing cell mass produced in the fermenter, water, citric acid, and unreacted ammonia and glucose. All streams may be assumed to be at 25°C, as are the contents of the well-mixed fermenter. The stoichiometry of the fermentation reaction is given by
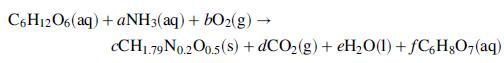
where the coefficients of the species (a, b, . . . ,) are to be determined. Experiments on the fermentation reaction have found that 70% of the glucose consumed is converted to citric acid and that the respiratory quotient (RQ) is 0.45 (RQ = moles of CO2 produced per mole of O2 consumed).
The following table gives data for selected process species. Information on other species may be found in Table B.1.
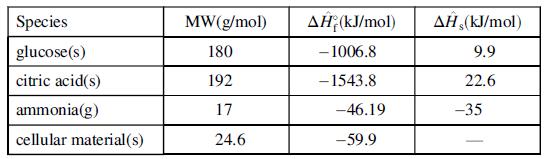
(a) Use elemental species balances to determine the coefficients in the stoichiometric equation.
(b) The system is sized so that 90% of the limiting reactant is consumed. For a citric acid production rate of 10 kg/h, estimate all stream and constituent flow rates in both kg/h and mol/h. What are the volumetric flow rates of air entering the fermenter and of the off-gas stream?
(c) The heats of formation for glucose and citric acid given in the above process description are for the species as solids, while the heat of formation of ammonia is for a gas. However, the fermentation reaction involves aqueous solutions of all three species. Show how Hess’s law can be used in estimating heat of formation in an aqueous solution from a heat of formation of either gaseous or solid species. Heats of solution may be assumed constant.
(d) Determine the rate at which heat must be added to or removed from (state which) the fermenter.
Exploratory Exercises—Research and Discover
(e) Various strains of A. niger exist, with some being useful in the production of specific chemicals, such as citric acid, and some being harmful. Provide a brief description of how it is to be cultured for the application in this problem.
(f) Identify safety issues associated with use of A. niger in the production of citric acid. Pick one of these issues and suggest means for mitigating risks in the process under consideration.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





