Consider the following isotope-exchange reaction: DCl (g) + HBr (g) DBr (g) + HCl (g) The amount
Question:
DCl (g) + HBr (g) ‡„ DBr (g) + HCl (g)
The amount of each species at equilibrium can be measured using proton and deuterium NMR (see Journal of Chemical Education 73 [1996]: 99). Using the spectroscopic information below, determine KP for this reaction at 298 K. For this reaction, Δε = 41 cmˆ’1, equal to the difference in zero-point energies between products versus reactants, and the ground-state electronic degeneracy is zero for all species.
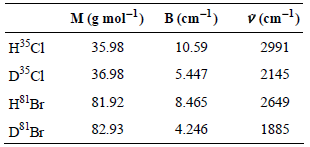
Transcribed Image Text:
M (g mol-l) в (сm-1) V (cm-1) н$C1 10.59 35.98 2991 D3°C1 36.98 5.447 2145 H$'Br 81.92 8.465 2649 D°'Br 82.93 1885 4.246
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
For DBr Notice that the vibrational partition function is approximately equal to on...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant Kc for the reaction is 4.2 at 1650°C. Initially 0.80 mol H2 and 0.80 mol CO2 are injected into a 5.0-L flask. Calculate the concentration of each species at equilibrium....
-
In the gas-phase reaction A + B ;:='0C + 2 D, it was found that, when 2.00 mol A, 1.00 mol B, and 3.00 mol D were mixed and allowed to come to equilibrium at 25C, the resulting mixture contained 0.79...
-
The equilibrium between hydrogen cyanide (HCN) and its isomer hydrogen isocyanide (HNC) is important in interstellar chemistry: HCN (g) HNC (g) A long-standing puzzle regarding this reaction is that...
-
Using the Trust Services Principles and Criteria for the Online Privacy Principle, develop an online privacy policy for Alltel Stadium that could be posted on the stadiums website for customers to...
-
1. How can you tell whether an ellipse is a circle from the equation? 2. Is the graph of x2 4y4 = 4 a hyperbola? Explain.
-
Benefits from which employer-provided plans will be received by the employee income-tax free? A. $7,000 in educational assistance. B. Group disability plan paid for by the employer. C. Prepaid legal...
-
Using any of the datasets that come with this text that include at least two quantitative variables and at least one categorical variable (or any other dataset that you find interesting and that...
-
Use Newtons method to find the coordinates of the inflection point of the curve y = e cos x, 0, < x < correct to six decimal places.
-
Effects of Adjusting Entries on the Accounting Equation Four adjusting entries are shown below. a. Interest Expense 1,595 Interest Payable 1,595 b. Interest Receivable 1,050 Interest Income 1,050 c....
-
Suppose Alice wants to communicate with Bob using symmetric key cryptography using a session key KS. In Section 8.2, we learned how public-key cryptography can be used to distribute the session key...
-
In Direct Measurement of the Size of the Helium Dimer by F. Luo, C. F. Geise, and W. R. Gentry [J. Chemical Physics 104 (1996): 1151], evidence for the helium dimer is presented. As one can imagine,...
-
The isotope exchange reaction for Cl 2 is as follows: 35 Cl 35 Cl + 37 Cl 37 Cl 237 Cl 35 Cl The equilibrium constant for this reaction is 4. Furthermore, the equilibrium constant for similar...
-
The octal numeration system refers to the base eight system, which uses the symbols 0, 1, 2, 3, 4, 5, 6, 7. Describe a process for converting octal numbers to decimal and decimal to octal.
-
Thomas transfers land worth $100,000 with an adjusted basis of $35,000 and a mortgage of $65,000 and equipment worth $30,000 and a basis of $10,000 to Andy Co. in return for 50 shares of stock....
-
The AB Corp. has been owned equally by individuals A and B for over five years. AB operates two qualified businesses, Q and R. The XYZ Corp. wishes to acquire Division Q, worth $500,000 (asset basis...
-
Falzone Company has two shareholders, Rita and Sal Corporation. Rita acquired her 300 shares in 2011 for $30,000 and Sal Corporation acquired its 200 shares in 2007 for $15,000. On August 2, 2019,...
-
Molly Caruso was the sole shareholder of Seneca Resorts Inc. Her basis in the stock was $200,000. Molly died when her stock was worth $5,000,000. Her gross estate, including the stock, was...
-
Prepare an exponential smoothing forecast.
-
A Frisbee is observed to fly nearly horizontally, which implies that the lift force must be approximately equal to the weight of the Frisbee. If the air speed over the top of the Frisbee is 9.0 m/s,...
-
In Problems, solve each system of equations. x + 2y + 3z = 5 y + 11z = 21 5y + 9z = 13
-
Identify the alkene that would yield the following products via ozonolysis: a. b. c. d.
-
Cyclopentanone was treated with lithium aluminum hydride followed by H 3 O + . Explain what you would look for in the IR spectrum of the product to verify that the expected reaction had occurred....
-
When 1-chlorobutane is treated with sodium hydroxide, two products are formed. Identify the two products, and explain how these products could be distinguished using IR spectroscopy.
-
Excerpts from Andre Company's December 31, 2024 and 2023, financial statements are presented below: Accounts receivable Inventory Net sales Cost of goods sold Total assets Net income Total...
-
The York City Hospital has just acquired new equipment. The equipment cost $ 4 , 2 5 0 , 0 0 0 , and the organization spent $ 1 3 5 , 0 0 0 on upgrading the physical plant to the new equipment will...
-
Calculate the following showing all the necessary steps: Note: CPP rate to be used in the calculation is 5.95%. Note: Don't forget to deduct pay period exemption\ \ Tanya earns $25.00 per hour. This...

Study smarter with the SolutionInn App


