Consider the following mechanism for ozone thermal decomposition: a. Derive the rate law expression for the loss
Question:
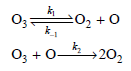
a. Derive the rate law expression for the loss of O3(g).
b. Under what conditions will the rate law expression for O3(g) decomposition be first order with respect to O3(g)?
Transcribed Image Text:
:02 +0 O3 03 +0- 202
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
a b Atomic oxygen is the intermediate species and the diffe...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following mechanism for renaturation of a double helix from its strands A and B: Derive the rate equation for the formation of the double helix and express the rate constant of the...
-
Consider the following mechanism for the thermal decomposition ofR2: Where R2, PA' PB are stable hydrocarbons and Rand R' are radicals. Find the dependence of the rate of decomposition of R, on the...
-
Consider the following mechanism for a reaction in aqueous solution and indicate the species acting as a catalyst: Explain why you believe this species is a catalyst. What is the overall reaction?...
-
Which of the following statements is correct regarding a work sheet and the adjustment process? Adjusting journal entries are prepared from the Adjusted Trial Balance columns. Adjusting journal...
-
Find the inverse function of f informally. Verify that f (f-1 (x)) = x and f-1(f (x)) = x. 1. f(x) = 6x 2. f(x) = 1/3x 3. f(x) = 3x + 1
-
Marian Plunket owns her own business and is considering an investment. If she undertakes the investment, it will pay $4000 at the end of each of the next three years. The opportunity requires an...
-
Let a random sample of 5 observations from a normal (, 2 ) distribution (where it is known that the mean = 25) be (a) What is the equation for the shape of the likelihood function of the variance ...
-
Colin Davis Machine Company maintains a general ledger account for each class of inventory, debiting such accounts for increases during the period and crediting them for decreases. The transactions...
-
a) Explain the following principles of valuation i. Principle of conformity ii. Principle of substitution iii. Principle of highest and best use iv. Principle of supply and demand v. Principle of...
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...
-
Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S²) through the following mechanism involving an intermediate helix (IH): a. Derive the rate law...
-
Determine the expression for fractional coverage as a function of pressure for the dissociative adsorption mechanism described in the text in which adsorption is accompanied by dissociation: R,(g) +...
-
Prove algebraically that the equation in Exercise 2d is an identity. In Exercise 2d tan x (cot x + tan x) = sec2x
-
The figure below shows a uniform electric field of magnitude E = 450 N/C making an angle of p = 64.0 with a flat surface of area A = 4.00 m. What is the magnitude of the electric flux through this...
-
Can you elucidate the molecular mechanisms underlying the regulation of respiratory enzymes, such as cytochrome c oxidase, and their role in maintaining cellular redox balance and energy production?
-
6. A firm has beginning retained earnings of $4200 and ending retained earnings of $4650.What is the amount of dividends paid of the firm earned a net income of $1950?
-
explain the interplay between respiratory metabolism and cellular signaling pathways, such as AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR), in regulating energy...
-
Tall Trees, Inc. is using the modified internal rate of return (MIRR) when evaluating projects. The company is able to reinvest cash flows received from the project at an annual rate of 10.34...
-
The E-field of an electromagnetic wave is described by Write an expression for the B-field. Determine vector B(0, 0). 3 (i + jEo sin (kz ot + /6)
-
After graduating from college and working a few years at a small technology firm. Preet scored a high-level job in the logistics department at Amex Corporation. Amex sells high-quality electronic...
-
Predict the products for each of the following reactions: a. b. c. d. . Ti[OCH(CH,),1. (+)-DET -- THCICH) (-)-DET
-
Benzoic acid, 1.35 g, is reacted with oxygen in a constant volume calorimeter to form H 2 O(l) and CO 2 (g) at 298 K. The mass of the water in the inner bath is 1.55 10 3 g. The temperature of the...
-
Calculate the P and T values for which Br2(g) is in a corresponding state to Xe(g) at 330. K and 72.0 bar.
-
Link to Digital Profile/Portfolio 2. You are taking a database snapshot of your RDS instance. What would be the impact to the I/O operations while taking snapshots? 3. What is the maximum size of RDS...
-
Define Divide and Conquer Run the simulation of merge sort in: https://www.hackerearth.com/practice/algorithms/sorting/merge-sort/visualize/ (not a question) Explain the algorithm of merge sort? Does...
-
Ask a non-IT person (your friend, child) how the Web is different from the Internet. Quote the most interesting part of their answer and then critique it based on what you know. Explain the process...

Study smarter with the SolutionInn App


