Consider the formation of glucose from carbon dioxide and water, that is, the reaction of the photosynthetic
Question:
6CO2(g) + 6H2O(l) †’ C6H12O6(s) + 6O2(g).
The following table of information will be useful in working this problem:
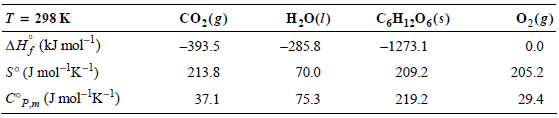
Calculate the entropy and enthalpy changes for this chemical system at T = 298 K and T = 310 K. Calculate also the entropy change of the surroundings and the universe at both temperatures, assuming that the system and surroundings are at the same temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





