Define a system and simplify the open-system energy balance (Equation 7.4-15) for each of the following cases.
Question:
Define a system and simplify the open-system energy balance (Equation 7.4-15) for each of the following cases. State when possible whether nonzero heat and shaft work terms are positive or negative. The solution of Part (a) is given as an illustration.
(a) Steam enters a rotary turbine and turns a shaft connected to a generator. The inlet and outlet steam ports are at the same height. Some energy is transferred to the surroundings as heat.
Solution. The system is the steam flowing from the inlet port to the outlet port:
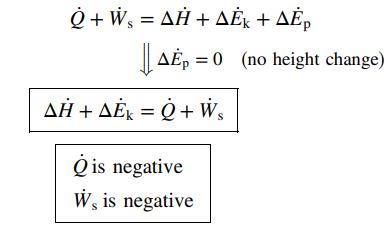
(b) A liquid stream flows through a heat exchanger in which it is heated from 25°C to 80°C. The inlet and outlet pipes have the same diameter, and there is no change in elevation between these points.
(c) Water passes through the sluice gate of a dam and falls on a turbine rotor, which turns a shaft connected to a generator. The fluid velocity on both sides of the dam is negligible, and the water undergoes insignificant pressure and temperature changes between the inlet and outlet.
(d) Crude oil is pumped through a cross-country pipeline. The pipe inlet is 200 m higher than the outlet, the pipe diameter is constant, and the pump is located near the midpoint of the pipeline. Energy dissipated by friction in the line is transferred as heat through the wall.
(e) A chemical reaction takes place in a continuous reactor that contains no moving parts. Kinetic and potential energy changes from inlet to outlet are negligible.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




